Harm, benefit and costs associated with low-dose glucocorticoids added to the treatment strategies for rheumatoid arthritis in elderly patients (GLORIA trial): study protocol for a randomised controlled trial
- PMID: 29370811
- PMCID: PMC5785876
- DOI: 10.1186/s13063-017-2396-3
Harm, benefit and costs associated with low-dose glucocorticoids added to the treatment strategies for rheumatoid arthritis in elderly patients (GLORIA trial): study protocol for a randomised controlled trial
Abstract
Background: Rheumatoid arthritis (RA) is a chronic inflammatory disease of the joints affecting 1% of the world population. It has major impact on patients through disability and associated comorbidities. Current treatment strategies have considerably improved the prognosis, but recent innovations (especially biologic drugs and the new class of so-called "JAK/STAT inhibitors") have important safety issues and are very costly. Glucocorticoids (GCs) are highly effective in RA, and could reduce the need for expensive treatment with biologic agents. However, despite more than 65 years of clinical experience, there is a lack of studies large enough to adequately document the benefit/harm balance. The result is inappropriate treatment strategies, i.e. both under-use and over-use of GCs, and consequently suboptimal treatment of RA.
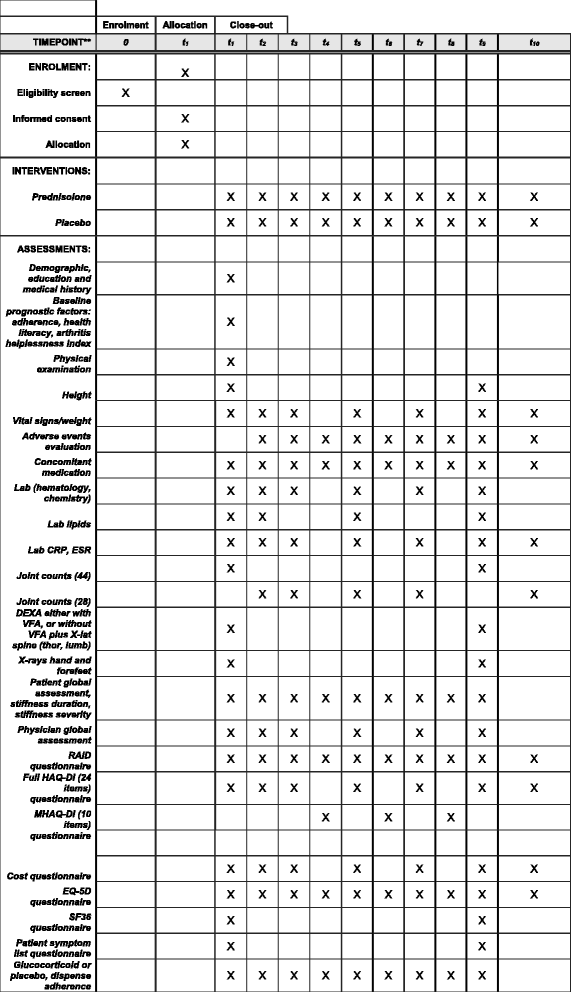
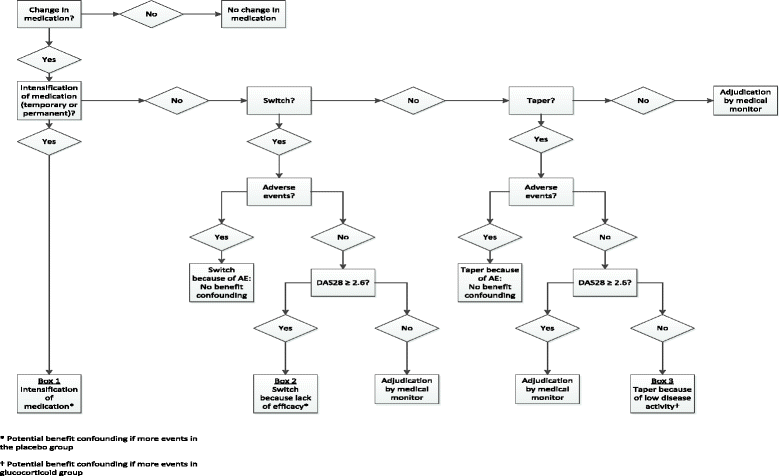
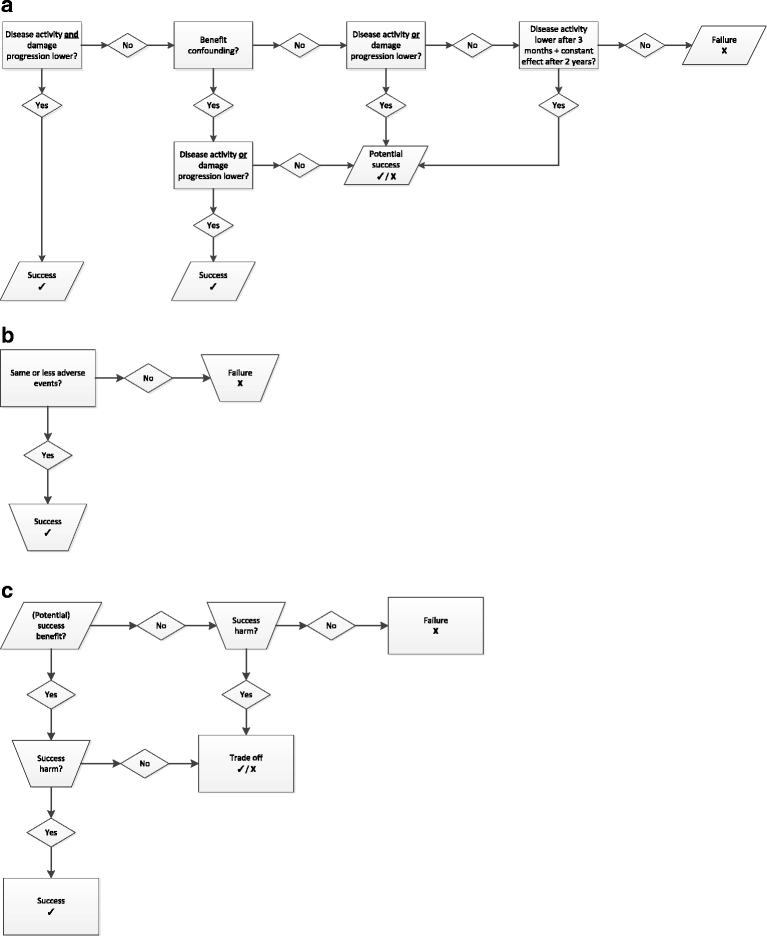
Methods: The GLORIA study is a pragmatic multicentre, 2-year, randomised, double-blind, clinical trial to assess the safety and effectiveness of a daily dose of 5 mg prednisolone or matching placebo added to standard of care in elderly patients with RA. Eligible participants are diagnosed with RA, have inadequate disease control (disease activity score, DAS28 ≥ 2.6), and are ≥ 65 years. The primary outcome measures are the time-averaged mean value of the DAS28 and the occurrence of serious adverse events or adverse events of special interest. During the trial, change in antirheumatic therapy is permitted as clinically indicated, except for GCs. Cost-effectiveness and cost-utility are secondary outcomes. The main challenge is the interpretation of the trial result with two primary endpoints and the pragmatic trial design that allows co-interventions. Another challenge is the definition of safety and the relative lack of power to detect differences between treatment groups. We have chosen to define safety as the number of patients experiencing at least one serious adverse event. We also specify a decision tree to guide our conclusion on the balance of benefit and harm, and our methodology to combat potential confounding caused by co-interventions.
Discussion: Pragmatic trials minimise impact on daily practice and maximise clinical relevance of the results, but analysis and interpretation of the results is challenging. We expect that the results of this trial are of importance for all rheumatologists who treat elderly patients with RA.
Trial registration: ClinicalTrials.gov, NCT02585258 . Registered on 20 October 2015.
Keywords: Benefit; Cost-effectiveness; Elderly; Glucocorticoids; Harm; Prednisolone; Rheumatoid arthritis; Safety.
Conflict of interest statement
Ethics approval and consent to participate
The study is carried out in compliance with the recommendations of the Declaration of Helsinki [27], GCP and in accordance with local guidelines, regulations, and acts. The medical ethical committee of all participating countries approved the trial (the names of the ethical bodies are provided in Additional file 4). Patients give informed consent before they are randomised in the study.
Consent for publication
The manuscript does not contain individual data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Buttgereit F, Mehta D, Kirwan J, Szechinski J, Boers M, Alten RE, Supronik J, Szombati I, Romer U, Witte S, Saag KG. Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2) Ann Rheum Dis. 2013;72:204–10. doi: 10.1136/annrheumdis-2011-201067. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

