Impact of question order on prioritisation of outcomes in the development of a core outcome set: a randomised controlled trial
- PMID: 29370827
- PMCID: PMC5784591
- DOI: 10.1186/s13063-017-2405-6
Impact of question order on prioritisation of outcomes in the development of a core outcome set: a randomised controlled trial
Abstract
Background: Core outcome set (COS) developers increasingly employ Delphi surveys to elicit stakeholders' opinions of which outcomes to measure and report in trials of a particular condition or intervention. Research outside of Delphi surveys and COS development demonstrates that question order can affect response rates and lead to 'context effects', where prior questions determine an item's meaning and influence responses. This study examined the impact of question order within a Delphi survey for a COS for oesophageal cancer surgery.
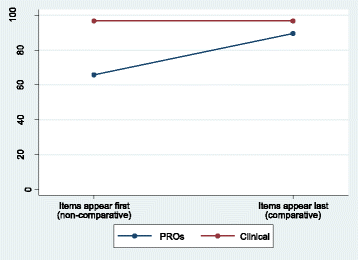
Methods: A randomised controlled trial was nested within the Delphi survey. Patients and health professionals were randomised to receive a survey including clinical and patient-reported outcomes (PROs), where the PRO section appeared first or last. Participants rated (1-9) the importance of 68 items for inclusion in a COS (ratings 7-9 considered 'essential'). Analyses considered the impact of question order on: (1) survey response rates; (2) participants' responses; and (3) items retained at end of the survey.
Results: In total, 116 patients and 71 professionals returned completed surveys. Question order did not affect response rates among patients, but fewer professionals responded when clinical items appeared first (difference = 31.3%, 95% confidence interval [CI] = 13.6-48.9%, P = 0.001). Question order led to different context effects within patients and professionals. While patients rated clinical items highly, irrespective of question order, more PROs were rated essential when appearing last rather than first (difference = 23.7%, 95% CI = 10.5-40.8%). Among professionals, the greatest impact was on clinical items; a higher percentage rated essential when appearing last (difference = 11.6%, 95% CI = 0.0-23.3%). An interaction between question order and the percentage of PRO/clinical items rated essential was observed for patients (P = 0.025) but not professionals (P = 0.357). Items retained for further consideration at the end of the survey were dependent on question order, with discordant items (retained by one question order group only) observed in patients (18/68 [26%]) and professionals (20/68 [29%]).
Conclusions: In the development of a COS, participants' ratings of potential outcomes within a Delphi survey depend on the context (order) in which the outcomes are asked, consequently impacting on the final COS. Initial piloting is recommended with consideration of the randomisation of items in the survey to reduce potential bias.
Trial registration: The randomised controlled trial reported within this paper was nested within the development of a core outcome set to investigate processes in core outcome set development. Outcomes were not health-related and trial registration was not therefore applicable.
Keywords: Context effects; Core outcome set; Delphi; Question order.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval for this study was granted by the South-West – Frenchay Research Ethics Committee (12/SW/0161). All patients were informed about the study and invited to participate. Only those returning a completed consent form were then posted a round 1 questionnaire. For professionals, a round 1 questionnaire was provided with initial study information and invitation to participate; in this instance the return of a completed questionnaire was deemed consent to participate, as agreed by the local ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous