Diffusion kurtosis imaging allows the early detection and longitudinal follow-up of amyloid-β-induced pathology
- PMID: 29370870
- PMCID: PMC6389136
- DOI: 10.1186/s13195-017-0329-8
Diffusion kurtosis imaging allows the early detection and longitudinal follow-up of amyloid-β-induced pathology
Abstract
Background: Alzheimer's disease (AD) is a progressive neurodegenerative disorder and the most common cause of dementia in the elderly population. In this study, we used the APP/PS1 transgenic mouse model to explore the feasibility of using diffusion kurtosis imaging (DKI) as a tool for the early detection of microstructural changes in the brain due to amyloid-β (Aβ) plaque deposition.
Methods: We longitudinally acquired DKI data of wild-type (WT) and APP/PS1 mice at 2, 4, 6 and 8 months of age, after which these mice were sacrificed for histological examination. Three additional cohorts of mice were also included at 2, 4 and 6 months of age to allow voxel-based co-registration between diffusion tensor and diffusion kurtosis metrics and immunohistochemistry.
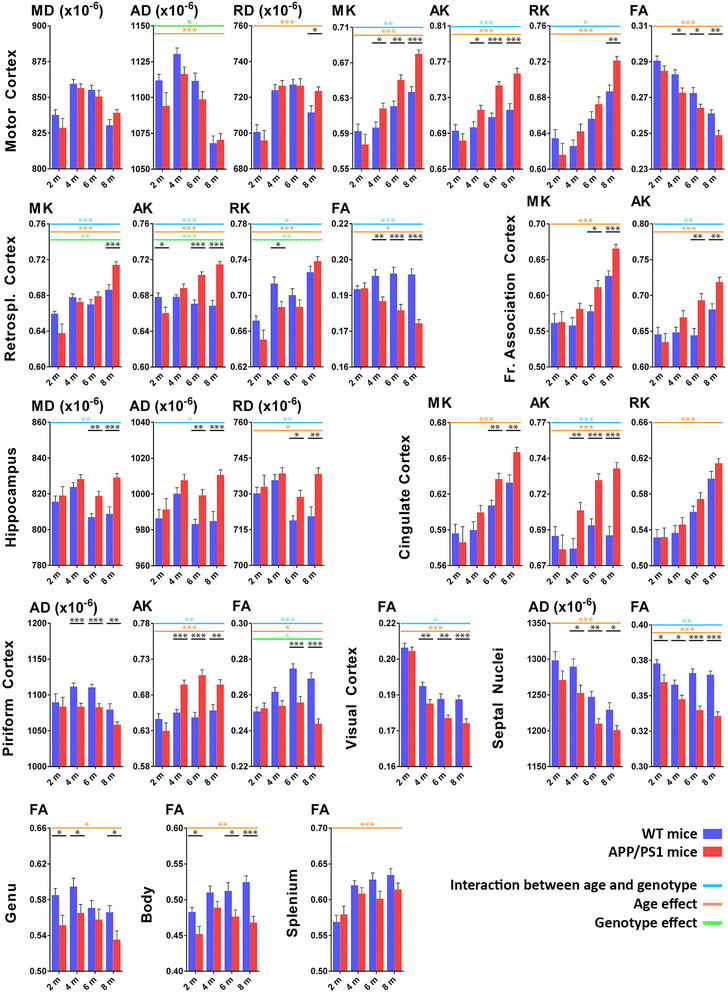
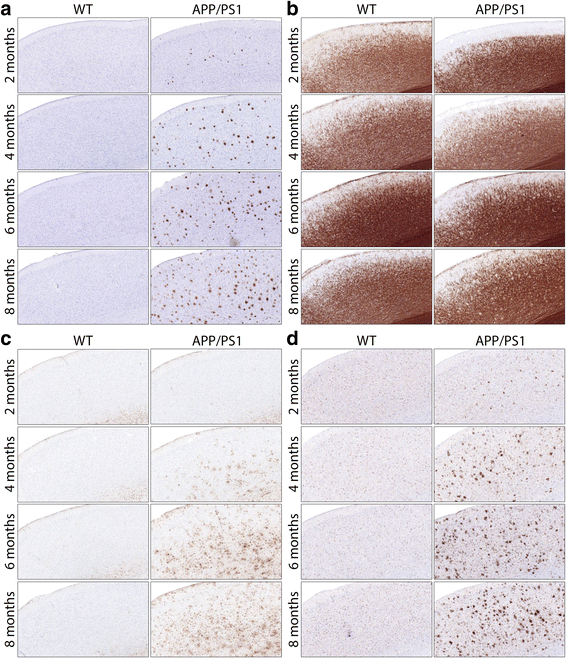
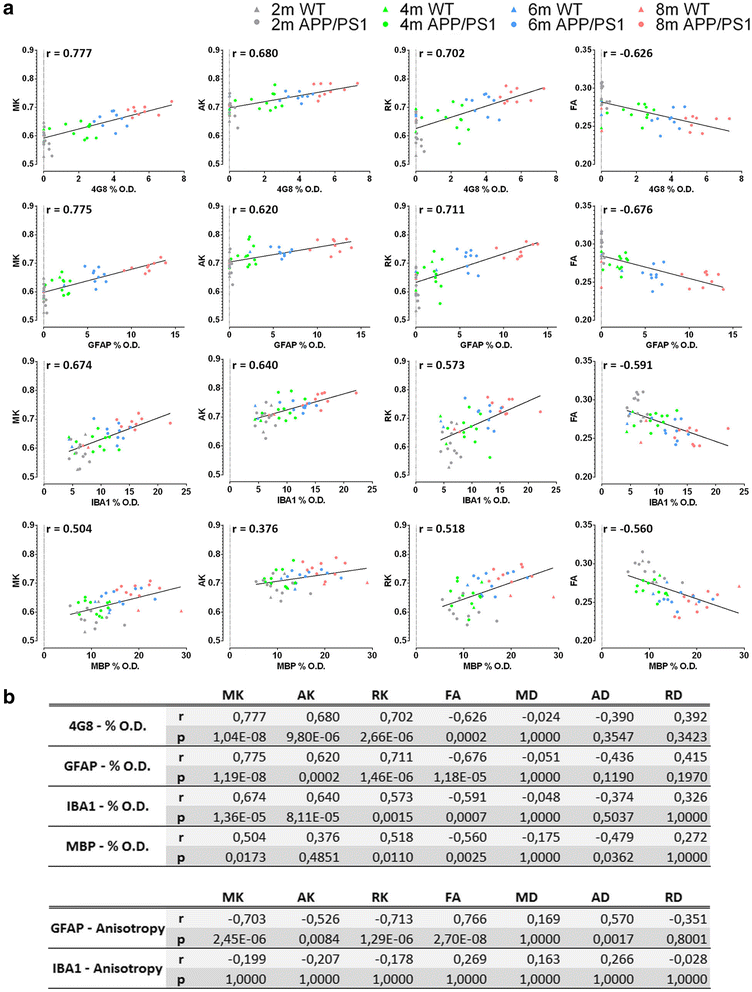
Results: Changes were observed in diffusion tensor (DT) and diffusion kurtosis (DK) metrics in many of the 23 regions of interest that were analysed. Mean and axial kurtosis were greatly increased owing to Aβ-induced pathological changes in the motor cortex of APP/PS1 mice at 4, 6 and 8 months of age. Additionally, fractional anisotropy (FA) was decreased in APP/PS1 mice at these respective ages. Linear discriminant analysis of the motor cortex data indicated that combining diffusion tensor and diffusion kurtosis metrics permits improved separation of WT from APP/PS1 mice compared with either diffusion tensor or diffusion kurtosis metrics alone. We observed that mean kurtosis and FA are the critical metrics for a correct genotype classification. Furthermore, using a newly developed platform to co-register the in vivo diffusion-weighted magnetic resonance imaging with multiple 3D histological stacks, we found high correlations between DK metrics and anti-Aβ (clone 4G8) antibody, glial fibrillary acidic protein, ionised calcium-binding adapter molecule 1 and myelin basic protein immunohistochemistry. Finally, we observed reduced FA in the septal nuclei of APP/PS1 mice at all ages investigated. The latter was at least partially also observed by voxel-based statistical parametric mapping, which showed significantly reduced FA in the septal nuclei, as well as in the corpus callosum, of 8-month-old APP/PS1 mice compared with WT mice.
Conclusions: Our results indicate that DKI metrics hold tremendous potential for the early detection and longitudinal follow-up of Aβ-induced pathology.
Keywords: APP/PS1; Alzheimer’s disease; Diffusion kurtosis imaging; Diffusion tensor imaging; Magnetic resonance imaging.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
All experimental procedures were performed in accordance with European guidelines (2010/63/EU) and were approved by the University of Antwerp Ethics Committee for Animal Experiments (approval number 2012-46).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- McKhann G, Knopman DS, Chertkow H, Hymann B, Jack CR, Jr, Kawas C, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

