Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials
- PMID: 29370992
- PMCID: PMC6126546
- DOI: 10.1016/S1470-2045(18)30006-8
Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials
Abstract
Background: Patients with metastatic sarcoma have limited treatment options. Nivolumab and ipilimumab are monoclonal antibodies targeting PD-1 and CTLA-4, respectively. We investigated the activity and safety of nivolumab alone or in combination with ipilimumab in patients with locally advanced, unresectable, or metastatic sarcoma.
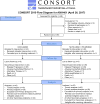
Methods: We did a multicentre, open-label, non-comparative, randomised, phase 2 study that enrolled patients aged 18 years or older and had central pathology confirmation of sarcoma with at least one measurable lesion by Response Evaluation Criteria In Solid Tumors (RECIST) 1.1, evidence of metastatic, locally advanced or unresectable disease, an ECOG performance status of 0-1, and received at least one previous line of systemic therapy. Patients were assigned to treatment in an unblinded manner, as this trial was conducted as two independent, non-comparative phase 2 trials. Enrolled patients were assigned (1:1) via a dynamic allocation algorithm to intravenous nivolumab 3 mg/kg every 2 weeks, or nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses. Thereafter, all patients received nivolumab monotherapy (3 mg/kg) every 2 weeks for up to 2 years. The primary endpoint was the proportion of patients with locally advanced, unresectable or metastatic soft tissue sarcoma achieving a confirmed objective response. Analysis was per protocol. This study is ongoing although enrolment is closed. It is registered with ClinicalTrials.gov, number NCT02500797.
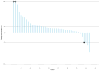
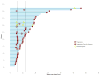
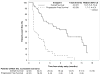
Findings: Between Aug 13, 2015, and March 17, 2016, 96 patients from 15 sites in the USA underwent central pathology review for eligibility and 85 eligible patients, including planned over-enrolment, were allocated to receive either nivolumab monotherapy (43 patients) or nivolumab plus ipilimumab (42 patients). The primary endpoint analysis was done according to protocol specifications in the first 76 eligible patients (38 patients per group). The number of confirmed responses was two (5% [92% CI 1-16] of 38 patients) in the nivolumab group and six (16% [7-30] of 38 patients) in the nivolumab plus ipilimumab group. The most common grade 3 or worse adverse events were anaemia (four [10%] patients), decreased lymphocyte count (three [7%]), and dehydration, increased lipase, pain, pleural effusion, respiratory failure, secondary benign neoplasm, and urinary tract obstruction (two [5%] patients each) among the 42 patients in the nivolumab group and anaemia (eight [19%] patients), hypotension (four [10%] patients), and pain and urinary tract infection (three [7%] patients each) among the 42 patients in the nivolumab plus ipilimumab group. Serious treatment-related adverse events occurred in eight (19%) of 42 patients receiving monotherapy and 11 (26%) of 42 patients receiving combination therapy, and included anaemia, anorexia, dehydration, decreased platelet count, diarrhoea, fatigue, fever, increased creatinine, increased alanine aminotransferase, increased aspartate aminotransferase, hyponatraemia, pain, pleural effusion, and pruritus. There were no treatment-related deaths.
Interpretation: Nivolumab alone does not warrant further study in an unselected sarcoma population given the limited efficacy. Nivolumab combined with ipilimumab demonstrated promising efficacy in certain sarcoma subtypes, with a manageable safety profile comparable to current available treatment options. The combination therapy met its predefined primary study endpoint; further evaluation of nivolumab plus ipilimumab in a randomised study is warranted.
Funding: Alliance Clinical Trials in Oncology, National Cancer Institute Cancer Therapy Evaluation Program, Bristol-Myers Squibb, Cycle for Survival.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
BAVT Grant funding from Merck and Pfizer, Consultant to Janseen, Lilly, Novartis and Karyppharm and Caris. Speaker for Lilly, Janseen and Caris. EH employee of Astellas 8/2016. WDT Personal fees from Eli Lilly, EMD Serono, Novartis, Eisai, Janseen, Immune design, Adaptimmune, Ariad, Daiichi Sankyo, Plexxikon, Morphotek, Advaxis, Tracon outside from submitted work. Patent for ATRX as a companion diagnostic for CDK4 inhibitors, pending. Patent for Drug discovery pending. Rest of authors have nothing to disclose.
Figures



Comment in
-
A start towards immunotherapy in sarcomas?Lancet Oncol. 2018 Mar;19(3):283-285. doi: 10.1016/S1470-2045(18)30039-1. Epub 2018 Jan 19. Lancet Oncol. 2018. PMID: 29370991 No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. - PubMed
-
- Seddon BWJ, Strauss SJ, Gordon Leahy M, Woll PJ, Cowie F, Rothermundt CA, Wood Z, Forsyth S, Khan I, Nash S, Patterson P, Beare S. GeDDiS: A prospective randomised controlled phase III trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft tissue sarcomas (EudraCT 2009-014907-29) Journal of Clinical Oncology. 2015:33.
-
- Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(8):786–93. - PMC - PubMed
-
- Schoffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629–37. - PubMed
-
- van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA007968/CA/NCI NIH HHS/United States
- P30 CA086862/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180836/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA077440/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- U10 CA047642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

