Complement activation predicts adverse pregnancy outcome in patients with systemic lupus erythematosus and/or antiphospholipid antibodies
- PMID: 29371202
- PMCID: PMC6037302
- DOI: 10.1136/annrheumdis-2017-212224
Complement activation predicts adverse pregnancy outcome in patients with systemic lupus erythematosus and/or antiphospholipid antibodies
Abstract
Objective: Studies in mouse models implicate complement activation as a causative factor in adverse pregnancy outcomes (APOs). We investigated whether activation of complement early in pregnancy predicts APOs in women with systemic lupus erythematosus (SLE) and/or antiphospholipid (aPL) antibodies.
Methods: The PROMISSE Study enrolled pregnant women with SLE and/or aPL antibodies (n=487) and pregnant healthy controls (n=204) at <12 weeks gestation and evaluated them monthly. APOs were: fetal/neonatal death, preterm delivery <36 weeks because of placental insufficiency or preeclampsia and/or growth restriction <5th percentile. Complement activation products were measured on serial blood samples obtained at each monthly visit.
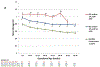
Results: APO occurred in 20.5% of SLE and/or aPL pregnancies. As early as 12-15 weeks, levels of Bb and sC5b-9 were significantly higher in patients with APOs and remained elevated through 31 weeks compared with those with normal outcomes. Moreover, Bb and sC5b-9 were significantly higher in patients with SLE and/or aPL without APOs compared with healthy controls. In logistic regression analyses, Bb and sC5b-9 at 12-15 weeks remained significantly associated with APO (ORadj=1.41 per SD increase; 95% CI 1.06 to 1.89; P=0.019 and ORadj=1.37 per SD increase; 95% CI 1.05 to 1.80; P=0.022, respectively) after controlling for demographic and clinical risk factors for APOs in PROMISSE. When analyses were restricted to patients with aPL (n=161), associations between Bb at 12-15 weeks and APOs became stronger (ORadj=2.01 per SD increase; 95% CI 1.16 to 3.49; P=0.013).
Conclusion: In pregnant patients with SLE and/or aPL, increased Bb and sC5b-9 detectable early in pregnancy are strongly predictive of APOs and support activation of complement, particularly the alternative pathway, as a contributor to APOs.
Keywords: antiphospholipid syndrome; complement; inflammation; pregnancy; systemic lupus erythematosus.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: DWB reports serving on the UCB Pharmaceuticals Advisory Board; JES has received an investigator-initiated grant from UCB Pharmaceuticals and consulting fees from Alnylam and Alexion.
Figures

References
-
- Qing X, Redecha PB, Burmeister MA, et al. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney international 2011;79:331–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

