The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension
- PMID: 29371380
- PMCID: PMC6383570
- DOI: 10.1183/13993003.01214-2017
The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension
Abstract
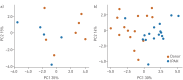
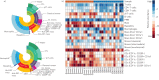
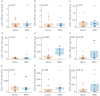
Increasing evidence points towards an inflammatory component underlying pulmonary hypertension. However, the conclusive characterisation of multiple inflammatory cell populations in the lung is challenging due to the complexity of marker specificity and tissue inaccessibility. We used an unbiased computational flow cytometry approach to delineate the inflammatory landscape of idiopathic pulmonary arterial hypertension (IPAH) and healthy donor lungs.Donor and IPAH samples were discriminated clearly using principal component analysis to reduce the multidimensional data obtained from single-cell flow cytometry analysis. In IPAH lungs, the predominant CD45+ cell type switched from neutrophils to CD3+ T-cells, with increases in CD4+, CD8+ and γδT-cell subsets. Additionally, diversely activated classical myeloid-derived dendritic cells (CD14-HLA-DR+CD11c+CD1a+/-) and nonclassical plasmacytoid dendritic cells (pDCs; CD14-CD11c-CD123+HLA-DR+), together with mast cells and basophils, were more abundant in IPAH samples. We describe, for the first time, the presence and regulation of two cell types in IPAH, γδT-cells and pDCs, which link innate and adaptive immunity.With our high-throughput flow cytometry with multidimensional dataset analysis, we have revealed the interactive interplay between multiple inflammatory cells is a crucial part of their integrative network. The identification of γδT-cells and pDCs in this disease potentially provides a missing link between IPAH, autoimmunity and inflammation.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: Disclosures can be found alongside this article at erj.ersjournals.com
Figures



Comment in
-
How does inflammation contribute to pulmonary hypertension?Eur Respir J. 2018 Jan 25;51(1):1702403. doi: 10.1183/13993003.02403-2017. Print 2018 Jan. Eur Respir J. 2018. PMID: 29371392 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
