Anti-Aspergillus Activities of the Respiratory Epithelium in Health and Disease
- PMID: 29371501
- PMCID: PMC5872311
- DOI: 10.3390/jof4010008
Anti-Aspergillus Activities of the Respiratory Epithelium in Health and Disease
Abstract
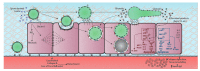
Respiratory epithelia fulfil multiple roles beyond that of gaseous exchange, also acting as primary custodians of lung sterility and inflammatory homeostasis. Inhaled fungal spores pose a continual antigenic, and potentially pathogenic, challenge to lung integrity against which the human respiratory mucosa has developed various tolerance and defence strategies. However, respiratory disease and immune dysfunction frequently render the human lung susceptible to fungal diseases, the most common of which are the aspergilloses, a group of syndromes caused by inhaled spores of Aspergillus fumigatus. Inhaled Aspergillus spores enter into a multiplicity of interactions with respiratory epithelia, the mechanistic bases of which are only just becoming recognized as important drivers of disease, as well as possible therapeutic targets. In this mini-review we examine current understanding of Aspergillus-epithelial interactions and, based upon the very latest developments in the field, we explore two apparently opposing schools of thought which view epithelial uptake of Aspergillus spores as either a curative or disease-exacerbating event.
Keywords: Aspergillus fumigatus; airway epithelial cells (AECs); epithelial responses; fungal pathogenesis; internalization; morphotypes; respiratory epithelium; spore uptake.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kleinkauf N., Verweij P., Arendrup M., Donnelly P., Cuenca-Estrella M., Fraaije B., Melchers W., Adriaenssens N., Kema G., Ullmann A. Risk Assessment on the Impact of Environmental Usage of Triazoles on the Development and Spread of Resistance to Medical Triazoles in Aspergillus Species. European Centre for Disease Prevention and Control (ECDC); Stockholm, Sweden: 2013.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

