Defining the role of the tumor vasculature in antitumor immunity and immunotherapy
- PMID: 29371595
- PMCID: PMC5833710
- DOI: 10.1038/s41419-017-0061-0
Defining the role of the tumor vasculature in antitumor immunity and immunotherapy
Abstract
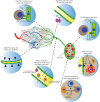
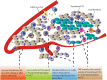
It is now well established that cancer cells co-exist within a complex environment with stromal cells and depend for their growth and dissemination on tight and plastic interactions with components of the tumor microenvironment (TME). Cancer cells incite the formation of new blood and lymphatic vessels from preexisting vessels to cope with their high nutrient/oxygen demand and favor tumor outgrowth. Research over the past decades has highlighted the crucial role played by tumor-associated blood and lymphatic vasculature in supporting immunoevasion and in subverting T-cell-mediated immunosurveillance, which are the main hallmarks of cancers. The structurally and functionally aberrant tumor vasculature contributes to the protumorigenic and immunosuppressive TME by maintaining a cancer cell's permissive environment characterized by hypoxia, acidosis, and high interstitial pressure, while simultaneously generating a physical barrier to T cells' infiltration. Recent research moreover has shown that blood endothelial cells forming the tumor vessels can actively suppress the recruitment, adhesion, and activity of T cells. Likewise, during tumorigenesis the lymphatic vasculature undergoes dramatic remodeling that facilitates metastatic spreading of cancer cells and immunosuppression. Beyond carcinogenesis, the erratic tumor vasculature has been recently implicated in mechanisms of therapy resistance, including those limiting the efficacy of clinically approved immunotherapies, such as immune checkpoint blockers and adoptive T-cell transfer. In this review, we discuss emerging evidence highlighting the major role played by tumor-associated blood and lymphatic vasculature in thwarting immunosurveillance mechanisms and antitumor immunity. Moreover, we also discuss novel therapeutic approaches targeting the tumor vasculature and their potential to help overcoming immunotherapy resistance.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

