AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation
- PMID: 29371602
- PMCID: PMC5785516
- DOI: 10.1038/s41467-017-02795-4
AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation
Abstract
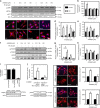
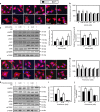
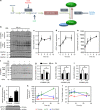
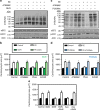
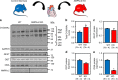
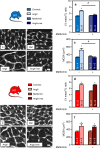
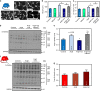
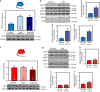
AMP-activated protein kinase (AMPK) has been shown to inhibit cardiac hypertrophy. Here, we show that submaximal AMPK activation blocks cardiomyocyte hypertrophy without affecting downstream targets previously suggested to be involved, such as p70 ribosomal S6 protein kinase, calcineurin/nuclear factor of activated T cells (NFAT) and extracellular signal-regulated kinases. Instead, cardiomyocyte hypertrophy is accompanied by increased protein O-GlcNAcylation, which is reversed by AMPK activation. Decreasing O-GlcNAcylation by inhibitors of the glutamine:fructose-6-phosphate aminotransferase (GFAT), blocks cardiomyocyte hypertrophy, mimicking AMPK activation. Conversely, O-GlcNAcylation-inducing agents counteract the anti-hypertrophic effect of AMPK. In vivo, AMPK activation prevents myocardial hypertrophy and the concomitant rise of O-GlcNAcylation in wild-type but not in AMPKα2-deficient mice. Treatment of wild-type mice with O-GlcNAcylation-inducing agents reverses AMPK action. Finally, we demonstrate that AMPK inhibits O-GlcNAcylation by mainly controlling GFAT phosphorylation, thereby reducing O-GlcNAcylation of proteins such as troponin T. We conclude that AMPK activation prevents cardiac hypertrophy predominantly by inhibiting O-GlcNAcylation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
AMP Kinase Activation Attenuates Cardiac Remodeling in Pulmonary Hypertension due to Heart Failure with Preserved Ejection Fraction; Lung Epithelial Progenitor Cells in Alveolar Regeneration; and Drug Discovery and Novel Therapies for Lung Cancer.Am J Respir Cell Mol Biol. 2019 Feb;60(2):244-247. doi: 10.1165/rcmb.2018-0280RO. Am J Respir Cell Mol Biol. 2019. PMID: 30476436 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

