Molecular Mechanism of Resveratrol's Lipid Membrane Protection
- PMID: 29371621
- PMCID: PMC5785473
- DOI: 10.1038/s41598-017-18943-1
Molecular Mechanism of Resveratrol's Lipid Membrane Protection
Abstract
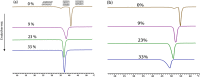
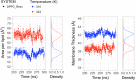
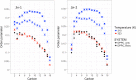
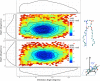
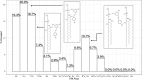
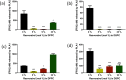
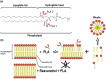
Resveratrol, a natural compound found in red wine and various vegetables, has drawn increasing interest due to its reported benefit in cardiovascular protection, neurodegenerative disorders, and cancer therapy. The mechanism by which resveratrol exerts such pleiotropic effects remains unclear. It remains as one of the most discussed polyphenol compounds in the debating "French Paradox". In this study, using molecular dynamics simulations of dipalmitoyl phosphatidylcholine (DPPC) bilayer with resveratrol, we generated a free energy map of resveratrol's location and orientation of inside the lipid bilayer. We found that resveratrol increases the surface area per lipid and decreases membrane thickness, which is the opposite effect of the well-studied cholesterol on liquid phase DPPC. Most importantly, based on the simulation observation that resveratrol has a high probability of forming hydrogen bonds with sn-1 and sn-2 ester groups, we discovered a new mechanism using experimental approach, in which resveratrol protects both sn-1 and sn-2 ester bonds of DPPC and distearoyl phosphatidylcholine (DSPC) from phospholipase A1 (PLA1) and phospholipase A2 (PLA2) cleavage. Our study elucidates the new molecular mechanism of potential health benefits of resveratrol and possibly other similar polyphenols and provides a new paradigm for drug design based on resveratrol and its analogs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
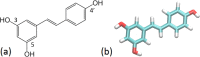


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

