Characterization of Nanodiamond-based anti-HIV drug Delivery to the Brain
- PMID: 29371638
- PMCID: PMC5785470
- DOI: 10.1038/s41598-017-16703-9
Characterization of Nanodiamond-based anti-HIV drug Delivery to the Brain
Abstract
Human Immunodeficiency Virus Type 1 (HIV-1) remains one of the leading causes of death worldwide. Present combination antiretroviral therapy has substantially improved HIV-1 related pathology. However, delivery of therapeutic agents to the HIV reservoir organ like Central nervous system (CNS) remains a major challenge primarily due to the ineffective transmigration of drugs through Blood Brain Barrier (BBB). The recent advent of nanomedicine-based drug delivery has stimulated the development of innovative systems for drug delivery. In this regard, particular focus has been given to nanodiamond due to its natural biocompatibility and non-toxic nature-making it a more efficient drug carrier than other carbon-based materials. Considering its potential and importance, we have characterized unmodified and surface-modified (-COOH and -NH2) nanodiamond for its capacity to load the anti-HIV-1 drug efavirenz and cytotoxicity, in vitro. Overall, our study has established that unmodified nanodiamond conjugated drug formulation has significantly higher drug loading capacity than surface-modified nanodiamond with minimum toxicity. Further, this nanodrug formulation was characterized by its drug dissolution profile, transmigration through the BBB, and its therapeutic efficacy. The present biological characterizations provide a foundation for further study of in-vivo pharmacokinetics and pharmacodynamics of nanodiamond-based anti-HIV drugs.
Conflict of interest statement
A US patent has been issued on this work (9616022B1).
Figures
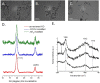
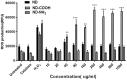
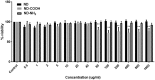
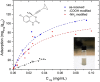
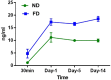

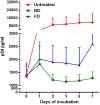
References
-
- Mellgren A, et al. Cerebrospinal fluid HIV-1 infection usually responds well to antiretroviral treatment. Antivir Ther. 2005;10:701–707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

