A link between Rel B expression and tumor progression in laryngeal cancer
- PMID: 29371965
- PMCID: PMC5768382
- DOI: 10.18632/oncotarget.23109
A link between Rel B expression and tumor progression in laryngeal cancer
Abstract
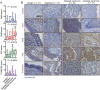
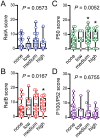
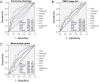
Laryngeal cancer is a frequent malignancy originating from the squamous vocal epithelium in a multi-stage fashion in response to environmental carcinogens. Although most cases can be cured by surgery and/or radiotherapy, advanced and relapsing disease is common, and biomarkers of such dismal cases are urgently needed. The cancer genome of laryngeal cancers was recently shown to feature a signature of aberrant nuclear factor (NF)-κB activation, but this finding has not been clinically exploited. We analyzed primary tumor samples of 96 well-documented and longitudinally followed patients covering the whole spectrum of laryngeal neoplasia, including 21 patients with benign laryngeal diseases, 15 patients with dysplasia, 43 patients with early-stage carcinoma, and 17 patients with locally advanced carcinoma, for immunoreactivity of RelA, RelB, P50, and P52/P100, the main NF-κB subunits that activate transcription. Results were cross-examined with indices of tumor progression and survival. Interestingly, RelB expression increased with tumor stage, grade, and local extent. Moreover, patients displaying high RelB immunoreactivity exhibited statistically significantly poorer survival compared with patients featuring low levels of RelB expression (P = 0.018 by log-rank test). Using Cox regression analyses and tumor stage, local extent, grade and RelA/RelB immunoreactivity, we develop a new score that can independently predict survival of patients with laryngeal cancer. Hence we provide a simple and affordable NF-κB-based test to predict prognosis in laryngeal cancer.
Keywords: biomarker; head and neck squamous cell carcinoma; immunohistochemistry; nuclear factor (NF)-κB; prognosis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures




Similar articles
-
Comprehensive Evaluation of Nuclear Factor-κΒ Expression Patterns in Non-Small Cell Lung Cancer.PLoS One. 2015 Jul 6;10(7):e0132527. doi: 10.1371/journal.pone.0132527. eCollection 2015. PLoS One. 2015. PMID: 26147201 Free PMC article.
-
[Function of alternative NF-κB activity in B-cell chronic lymphocytic leukemia cells].Zhonghua Xue Ye Xue Za Zhi. 2014 Jan;35(1):40-5. doi: 10.3760/cma.j.issn.0253-2727.2014.01.011. Zhonghua Xue Ye Xue Za Zhi. 2014. PMID: 24602731 Chinese.
-
The pivotal role of the alternative NF-kappaB pathway in maintenance of basal bone homeostasis and osteoclastogenesis.J Bone Miner Res. 2010 Apr;25(4):809-18. doi: 10.1359/jbmr.091030. J Bone Miner Res. 2010. PMID: 19839765
-
[NF-κB subunits regulate maspin expression in prostate cancer cells in vitro].Zhonghua Zhong Liu Za Zhi. 2012 Mar;34(3):165-8. doi: 10.3760/cma.j.issn.0253-3766.2012.03.002. Zhonghua Zhong Liu Za Zhi. 2012. PMID: 22780967 Chinese.
-
Immunohistochemical analysis of NF-kappaB signaling proteins IKKepsilon, p50/p105, p52/p100 and RelA in prostate cancers.APMIS. 2009 Aug;117(8):623-8. doi: 10.1111/j.1600-0463.2009.02506.x. APMIS. 2009. PMID: 19664134
Cited by
-
Insights into expression and localization of HPV16 LCR-associated transcription factors and association with LCR activity in HNSCC.Mol Ther Oncol. 2024 Dec 21;33(1):200926. doi: 10.1016/j.omton.2024.200926. eCollection 2025 Mar 20. Mol Ther Oncol. 2024. PMID: 39886356 Free PMC article.
-
Constitutive activation of NF-κB inducing kinase (NIK) in the mesenchymal lineage using Osterix (Sp7)- or Fibroblast-specific protein 1 (S100a4)-Cre drives spontaneous soft tissue sarcoma.PLoS One. 2021 Jul 22;16(7):e0254426. doi: 10.1371/journal.pone.0254426. eCollection 2021. PLoS One. 2021. PMID: 34292968 Free PMC article.
-
Biological characteristics of transcription factor RelB in different immune cell types: implications for the treatment of multiple sclerosis.Mol Brain. 2019 Dec 27;12(1):115. doi: 10.1186/s13041-019-0532-6. Mol Brain. 2019. PMID: 31881915 Free PMC article. Review.
-
Regulation of NFκB Signalling by Ubiquitination: A Potential Therapeutic Target in Head and Neck Squamous Cell Carcinoma?Cancers (Basel). 2020 Oct 7;12(10):2877. doi: 10.3390/cancers12102877. Cancers (Basel). 2020. PMID: 33036368 Free PMC article. Review.
References
-
- Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 017;3:524–548. https://doi.org/10.1001/jamaoncol.2016.5688. - DOI - PMC - PubMed
-
- Chatenoud L, Garavello W, Pagan E, Bertuccio P, Gallus S, La Vecchia C, Negri E, Bosetti C. Laryngeal cancer mortality trends in European countries. Int J Cancer. 2016;138:833–842. https://doi.org/10.1002/ijc.29833. - DOI - PubMed
-
- Groome PA, O’Sullivan B, Irish JC, Rothwell DM, Schulze K, Warde PR, Schneider KM, Mackenzie RG, Hodson DI, Hammond JA, Gulavita SP, Eapen LJ, Dixon PF, et al. Management and outcome differences in supraglottic cancer between Ontario, Canada, and the Surveillance, Epidemiology, and End Results areas of the United States. J ClinOncol. 2003;21:496–505. https://doi.org/10.1200/JCO.2003.10.106. - DOI - PubMed
-
- Peng WJ, Mi J, Jiang YH. Asbestos exposure and laryngeal cancer mortality. Laryngoscope. 2016;126:1169–1174. https://doi.org/10.1002/lary.25693. - DOI - PubMed
-
- Di Maso M, Talamini R, Bosetti C, Montella M, Zucchetto A, Libra M, Negri E, Levi F, La Vecchia C, Franceschi S, Serraino D, Polesel J. Red meat and cancer risk in a network of case-control studies focusing on cooking practices. Ann Oncol. 2013;24:3107–3112. https://doi.org/10.1093/annonc/mdt392. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

