Comparison Between Adjuvant and Early-Salvage Postprostatectomy Radiotherapy for Prostate Cancer With Adverse Pathological Features
- PMID: 29372236
- PMCID: PMC5885162
- DOI: 10.1001/jamaoncol.2017.5230
Comparison Between Adjuvant and Early-Salvage Postprostatectomy Radiotherapy for Prostate Cancer With Adverse Pathological Features
Erratum in
-
Data Presentation Error in Results Paragraph of Abstract.JAMA Oncol. 2018 May 1;4(5):751. doi: 10.1001/jamaoncol.2018.0302. JAMA Oncol. 2018. PMID: 29470580 Free PMC article. No abstract available.
Abstract
Importance: Prostate cancer with adverse pathological features (ie, pT3 and/or positive margins) after prostatectomy may be managed with adjuvant radiotherapy (ART) or surveillance followed by early-salvage radiotherapy (ESRT) for biochemical recurrence. The optimal timing of postoperative radiotherapy is unclear.
Objective: To compare the clinical outcomes of postoperative ART and ESRT administered to patients with prostate cancer with adverse pathological features.
Design, setting, and participants: This multi-institutional, propensity score-matched cohort study involved 1566 consecutive patients who underwent postprostatectomy ART or ESRT at 10 US academic medical centers between January 1, 1987, and December 31, 2013. Propensity score 1-to-1 matching was used to account for covariates potentially associated with treatment selection. Data were collected from January 1 to September 30, 2016. Data analysis was conducted from October 1, 2016, to October 21, 2017.
Main outcomes and measures: Freedom from postirradiation biochemical failure, freedom from distant metastases, and overall survival. All outcomes were measured from date of surgery to address lead-time bias.
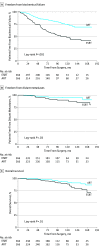
Results: Of 1566 patients, 1195 with prostate-specific antigen levels of 0.1 to 0.5 ng/mL received ESRT and 371 patients with prostate-specific antigen levels lower than 0.1 ng/mL received ART. The median age (interquartile range) was 60 (55-65) years. After propensity score matching, the median (interquartile range) follow-up after surgery was similar between the ESRT and ART groups (73.3 [44.9-106.6] months vs 65.8 [40-107] months; P = .22). Adjuvant RT, compared with ESRT, was associated with higher freedom from biochemical failure (12-year actuarial rates: 69% [95% CI, 60%-76%] vs 43% [95% CI, 35%-51%]; effect size, 26%), freedom from distant metastases (95% [95% CI, 90%-97%] vs 85% [95% CI, 76%-90%]; effect size, 10%), and overall survival (91% [95% CI, 84%-95%] vs 79% [95% CI, 69%-86%]; effect size, 12%). Adjuvant RT, lower Gleason score and T stage, nodal irradiation, and postoperative androgen deprivation therapy were favorable prognostic features on multivariate analysis for biochemical failure. Sensitivity analysis demonstrated that the decreased risk of biochemical failure associated with ART remained significant unless more than 56% of patients in the ART group were cured by surgery alone. This threshold is greater than the estimated 12-year freedom from biochemical failure rate of 33% to 52% after radical prostatectomy alone, as determined by a contemporary dynamic nomogram.
Conclusions and relevance: Adjuvant RT, compared with ESRT, was associated with reduced biochemical recurrence, distant metastases, and death for high-risk patients, pending prospective validation. These findings suggest that a greater proportion of patients with prostate cancer who have adverse pathological features may benefit from postprostatectomy ART rather than surveillance followed by ESRT.
Conflict of interest statement
Figures
Comment in
-
Re: Comparison between Adjuvant and Early-Salvage Postprostatectomy Radiotherapy for Prostate Cancer with Adverse Pathological Features.J Urol. 2018 Sep;200(3):497-499. doi: 10.1016/j.juro.2018.05.115. Epub 2018 May 30. J Urol. 2018. PMID: 30412979 No abstract available.
-
Comparing Adjuvant vs Early-Salvage Radiotherapy After Radical Prostatectomy.JAMA Oncol. 2018 Nov 1;4(11):1618-1619. doi: 10.1001/jamaoncol.2018.3533. JAMA Oncol. 2018. PMID: 30422245 No abstract available.
-
Comparing Adjuvant vs Early-Salvage Radiotherapy After Radical Prostatectomy.JAMA Oncol. 2018 Nov 1;4(11):1619-1620. doi: 10.1001/jamaoncol.2018.3536. JAMA Oncol. 2018. PMID: 30422246 No abstract available.
-
Comparing Adjuvant vs Early-Salvage Radiotherapy After Radical Prostatectomy.JAMA Oncol. 2018 Nov 1;4(11):1620. doi: 10.1001/jamaoncol.2018.3539. JAMA Oncol. 2018. PMID: 30422247 No abstract available.
-
Comparing Adjuvant vs Early-Salvage Radiotherapy After Radical Prostatectomy-Reply.JAMA Oncol. 2018 Nov 1;4(11):1620-1621. doi: 10.1001/jamaoncol.2018.3552. JAMA Oncol. 2018. PMID: 30422248 No abstract available.
-
Postoperative Radiation Therapy in Localized Prostate Cancer: When, How Much, and How Fast?Int J Radiat Oncol Biol Phys. 2019 Feb 1;103(2):289-292. doi: 10.1016/j.ijrobp.2018.08.003. Int J Radiat Oncol Biol Phys. 2019. PMID: 30647001 No abstract available.
References
-
- Hager B, Kraywinkel K, Keck B, et al. Increasing use of radical prostatectomy for locally advanced prostate cancer in the USA and Germany: a comparative population-based study. Prostate Cancer Prostatic Dis. 2017;20(1):61-66. - PubMed
-
- Karakiewicz PI, Eastham JA, Graefen M, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66(6):1245-1250. - PubMed
-
- Thompson IM Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296(19):2329-2335. - PubMed
-
- Briganti A, Wiegel T, Joniau S, et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol. 2012;62(3):472-487. - PubMed
-
- Bolla M, van Poppel H, Tombal B, et al. ; European Organisation for Research and Treatment of Cancer, Radiation Oncology and Genito-Urinary Groups . Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380(9858):2018-2027. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical