Association of Intracerebral Hemorrhage Among Patients Taking Non-Vitamin K Antagonist vs Vitamin K Antagonist Oral Anticoagulants With In-Hospital Mortality
- PMID: 29372247
- PMCID: PMC5839299
- DOI: 10.1001/jama.2017.21917
Association of Intracerebral Hemorrhage Among Patients Taking Non-Vitamin K Antagonist vs Vitamin K Antagonist Oral Anticoagulants With In-Hospital Mortality
Abstract
Importance: Although non-vitamin K antagonist oral anticoagulants (NOACs) are increasingly used to prevent thromboembolic disease, there are limited data on NOAC-related intracerebral hemorrhage (ICH).
Objective: To assess the association between preceding oral anticoagulant use (warfarin, NOACs, and no oral anticoagulants [OACs]) and in-hospital mortality among patients with ICH.
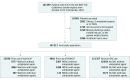
Design, setting, and participants: Retrospective cohort study of 141 311 patients with ICH admitted from October 2013 to December 2016 to 1662 Get With The Guidelines-Stroke hospitals.
Exposures: Anticoagulation therapy before ICH, defined as any use of OACs within 7 days prior to hospital arrival.
Main outcomes and measures: In-hospital mortality.
Results: Among 141 311 patients with ICH (mean [SD] age, 68.3 [15.3] years; 48.1% women), 15 036 (10.6%) were taking warfarin and 4918 (3.5%) were taking NOACs preceding ICH, and 39 585 (28.0%) and 5783 (4.1%) were taking concomitant single and dual antiplatelet agents, respectively. Patients with prior use of warfarin or NOACs were older and had higher prevalence of atrial fibrillation and prior stroke. Acute ICH stroke severity (measured by the National Institutes of Health Stroke Scale) was not significantly different across the 3 groups (median, 9 [interquartile range, 2-21] for warfarin, 8 [2-20] for NOACs, and 8 [2-19] for no OACs). The unadjusted in-hospital mortality rates were 32.6% for warfarin, 26.5% for NOACs, and 22.5% for no OACs. Compared with patients without prior use of OACs, the risk of in-hospital mortality was higher among patients with prior use of warfarin (adjusted risk difference [ARD], 9.0% [97.5% CI, 7.9% to 10.1%]; adjusted odds ratio [AOR], 1.62 [97.5% CI, 1.53 to 1.71]) and higher among patients with prior use of NOACs (ARD, 3.3% [97.5% CI, 1.7% to 4.8%]; AOR, 1.21 [97.5% CI, 1.11-1.32]). Compared with patients with prior use of warfarin, patients with prior use of NOACs had a lower risk of in-hospital mortality (ARD, -5.7% [97.5% CI, -7.3% to -4.2%]; AOR, 0.75 [97.5% CI, 0.69 to 0.81]). The difference in mortality between NOAC-treated patients and warfarin-treated patients was numerically greater among patients with prior use of dual antiplatelet agents (32.7% vs 47.1%; ARD, -15.0% [95.5% CI, -26.3% to -3.8%]; AOR, 0.50 [97.5% CI, 0.29 to 0.86]) than among those taking these agents without prior antiplatelet therapy (26.4% vs 31.7%; ARD, -5.0% [97.5% CI, -6.8% to -3.2%]; AOR, 0.77 [97.5% CI, 0.70 to 0.85]), although the interaction P value (.07) was not statistically significant.
Conclusions and relevance: Among patients with ICH, prior use of NOACs or warfarin was associated with higher in-hospital mortality compared with no OACs. Prior use of NOACs, compared with prior use of warfarin, was associated with lower risk of in-hospital mortality.
Conflict of interest statement
Figures

References
-
- Marzec LN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69(20):2475-2484. - PubMed
-
- Gadsbøll K, Staerk L, Fosbøl EL, et al. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J. 2017;38(12):899-906. - PubMed
-
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. - PubMed
-
- Granger CB, Alexander JH, McMurray JJ, et al. ; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. - PubMed
-
- Patel MR, Mahaffey KW, Garg J, et al. ; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

