Genome Mining and Assembly-Line Biosynthesis of the UCS1025A Pyrrolizidinone Family of Fungal Alkaloids
- PMID: 29373009
- PMCID: PMC5817883
- DOI: 10.1021/jacs.8b00056
Genome Mining and Assembly-Line Biosynthesis of the UCS1025A Pyrrolizidinone Family of Fungal Alkaloids
Abstract
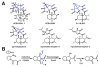
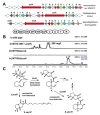
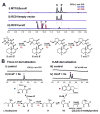
UCS1025A is a fungal polyketide/alkaloid that displays strong inhibition of telomerase. The structures of UCS1025A and related natural products are featured by a tricyclic furopyrrolizidine connected to a trans-decalin fragment. We mined the genome of a thermophilic fungus and activated the ucs gene cluster to produce UCS1025A at a high titer. Genetic and biochemical analysis revealed a PKS-NRPS assembly line that activates 2S,3S-methylproline derived from l-isoleucine, followed by Knoevenagel condensation to construct the pyrrolizidine moiety. Oxidation of the 3S-methyl group to a carboxylate leads to an oxa-Michael cyclization and furnishes the furopyrrolizidine. Our work reveals a new strategy used by nature to construct heterocyclic alkaloid-like ring systems using assembly line logic.
Figures

Similar articles
-
Genetic manipulation of the Fusarium fujikuroi fusarin gene cluster yields insight into the complex regulation and fusarin biosynthetic pathway.Chem Biol. 2013 Aug 22;20(8):1055-66. doi: 10.1016/j.chembiol.2013.07.004. Epub 2013 Aug 8. Chem Biol. 2013. PMID: 23932525
-
Pyranonigrin E: a PKS-NRPS hybrid metabolite from Aspergillus niger identified by genome mining.Chembiochem. 2013 Nov 4;14(16):2095-9. doi: 10.1002/cbic.201300430. Epub 2013 Sep 17. Chembiochem. 2013. PMID: 24106156
-
Genome Mining Reveals Biosynthesis of the Antifungal Lipopeptaibols, Texenomycins, through a Hybrid PKS-NRPS System, in the Fungus Mariannaea elegans.J Agric Food Chem. 2025 Jan 8;73(1):226-236. doi: 10.1021/acs.jafc.4c08847. Epub 2024 Dec 20. J Agric Food Chem. 2025. PMID: 39704078
-
[The PKS/NRPS hetero-gene cluster of epothilones].Sheng Wu Gong Cheng Xue Bao. 2003 Sep;19(5):511-5. Sheng Wu Gong Cheng Xue Bao. 2003. PMID: 15969075 Review. Chinese.
-
Natural products from thioester reductase containing biosynthetic pathways.Nat Prod Rep. 2018 Sep 19;35(9):847-878. doi: 10.1039/c8np00013a. Nat Prod Rep. 2018. PMID: 29916519 Free PMC article. Review.
Cited by
-
Enzymatic cis-Decalin Formation in Natural Product Biosynthesis.J Am Chem Soc. 2023 Feb 15;145(6):3301-3305. doi: 10.1021/jacs.2c12854. Epub 2023 Feb 1. J Am Chem Soc. 2023. PMID: 36723171 Free PMC article.
-
Enzymatic Intermolecular Hetero-Diels-Alder Reaction in the Biosynthesis of Tropolonic Sesquiterpenes.J Am Chem Soc. 2019 Sep 11;141(36):14052-14056. doi: 10.1021/jacs.9b06592. Epub 2019 Sep 3. J Am Chem Soc. 2019. PMID: 31461283 Free PMC article.
-
Deciphering chemical logic of fungal natural product biosynthesis through heterologous expression and genome mining.Nat Prod Rep. 2023 Jan 25;40(1):89-127. doi: 10.1039/d2np00050d. Nat Prod Rep. 2023. PMID: 36125308 Free PMC article. Review.
-
Recent Approaches for Downplaying Antibiotic Resistance: Molecular Mechanisms.Biomed Res Int. 2023 Jan 23;2023:5250040. doi: 10.1155/2023/5250040. eCollection 2023. Biomed Res Int. 2023. PMID: 36726844 Free PMC article. Review.
-
Harnessing the biocatalytic potential of iron- and α-ketoglutarate-dependent dioxygenases in natural product total synthesis.Nat Prod Rep. 2020 Aug 1;37(8):1065-1079. doi: 10.1039/c9np00075e. Epub 2020 Feb 14. Nat Prod Rep. 2020. PMID: 32055818 Free PMC article. Review.
References
-
- Neuman MG, Cohen L, Opris M, Nanau RM, Hyunjin J. J Pharm Pharm Sci. 2015;18:825–843. - PubMed
-
- Nicolaou KC, Pulukuri KK, Rigol S, Buchman M, Shah AA, Cen N, McCurry MD, Beabout K, Shamoo Y. J Am Chem Soc. 2017;139:15868–15877. - PMC - PubMed
- Nicolaou KC, Shah AA, Korman H, Khan T, Shi L, Worawalai W, Theodorakis EA. Angew Chem Int Ed Engl. 2015;54:9203–9208. - PMC - PubMed
- Uchida K, Ogawa T, Yasuda Y, Mimura H, Fujimoto T, Fukuyama T, Wakimoto T, Asakawa T, Hamashima Y, Kan T. Angew Chem Int Ed Engl. 2012;51:12850–12853. - PubMed
- de Figueiredo RM, Fröhlich R, Christmann M. Angew Chem Int Ed Engl. 2007;46:2883–2886. - PubMed
- Hoye TR, Dvornikovs V. J Am Chem Soc. 2006;128:2550–2551. - PubMed
- Lambert TH, Danishefsky SJ. J Am Chem Soc. 2006;128:426–427. - PubMed
-
- Li G, Kusari S, Spiteller M. Nat Prod Rep. 2014;31:1175–1201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

