Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer
- PMID: 29373071
- PMCID: PMC5858523
- DOI: 10.1200/JCO.2016.71.3495
Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer
Abstract
Purpose Studies suggest that a subset of patients with triple-negative breast cancer (TNBC) have tumors that express the androgen receptor (AR) and may benefit from an AR inhibitor. This phase II study evaluated the antitumor activity and safety of enzalutamide in patients with locally advanced or metastatic AR-positive TNBC. Patients and Methods Tumors were tested for AR with an immunohistochemistry assay optimized for breast cancer; nuclear AR staining > 0% was considered positive. Patients received enzalutamide 160 mg once per day until disease progression. The primary end point was clinical benefit rate (CBR) at 16 weeks. Secondary end points included CBR at 24 weeks, progression-free survival, and safety. End points were analyzed in all enrolled patients (the intent-to-treat [ITT] population) and in patients with one or more postbaseline assessment whose tumor expressed ≥ 10% nuclear AR (the evaluable subgroup). Results Of 118 patients enrolled, 78 were evaluable. CBR at 16 weeks was 25% (95% CI, 17% to 33%) in the ITT population and 33% (95% CI, 23% to 45%) in the evaluable subgroup. Median progression-free survival was 2.9 months (95% CI, 1.9 to 3.7 months) in the ITT population and 3.3 months (95% CI, 1.9 to 4.1 months) in the evaluable subgroup. Median overall survival was 12.7 months (95% CI, 8.5 months to not yet reached) in the ITT population and 17.6 months (95% CI, 11.6 months to not yet reached) in the evaluable subgroup. Fatigue was the only treatment-related grade 3 or higher adverse event with an incidence of > 2%. Conclusion Enzalutamide demonstrated clinical activity and was well tolerated in patients with advanced AR-positive TNBC. Adverse events related to enzalutamide were consistent with its known safety profile. This study supports additional development of enzalutamide in advanced TNBC.
Trial registration: ClinicalTrials.gov NCT01889238.
Figures
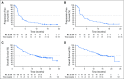
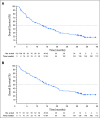
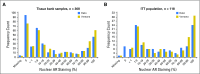
Comment in
-
AR Inhibition Achieves Responses in AR+ Triple-Negative Breast Cancer.Cancer Discov. 2018 Apr;8(4):OF8. doi: 10.1158/2159-8290.CD-RW2018-022. Epub 2018 Feb 9. Cancer Discov. 2018. PMID: 29439153
-
Is the Clinical Benefit Rate at Sixteen Weeks a Reliable End Point?J Clin Oncol. 2018 Aug 10;36(23):2457-2458. doi: 10.1200/JCO.2018.78.5139. Epub 2018 May 30. J Clin Oncol. 2018. PMID: 29847293 No abstract available.
-
Reply to K.S. Shohdy et al.J Clin Oncol. 2018 Aug 10;36(23):2458-2459. doi: 10.1200/JCO.2018.78.8349. Epub 2018 May 30. J Clin Oncol. 2018. PMID: 29847296 No abstract available.
References
-
- Kast K, Link T, Friedrich K, et al. : Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat 150:621-629, 2015 - PubMed
-
- Thomas ES, Gomez HL, Li RK, et al. : Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 25:5210-5217, 2007 - PubMed
-
- von Minckwitz G, Puglisi F, Cortes J, et al. : Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): An open-label, randomised phase 3 trial. Lancet Oncol 15:1269-1278, 2014 - PubMed
-
- Gerratana L, Fanotto V, Bonotto M, et al. : Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis 32:125-133, 2015 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

