Thalamic Control of Cognition and Social Behavior Via Regulation of Gamma-Aminobutyric Acidergic Signaling and Excitation/Inhibition Balance in the Medial Prefrontal Cortex
- PMID: 29373121
- PMCID: PMC5862785
- DOI: 10.1016/j.biopsych.2017.11.033
Thalamic Control of Cognition and Social Behavior Via Regulation of Gamma-Aminobutyric Acidergic Signaling and Excitation/Inhibition Balance in the Medial Prefrontal Cortex
Abstract
Background: The mediodorsal thalamus plays a critical role in cognition through its extensive innervation of the medial prefrontal cortex (mPFC), but how the two structures cooperate at the single-cell level to generate associated cognitive functions and other mPFC-dependent behaviors remains elusive. Maintaining the proper balance between excitation and inhibition (E/I balance) is of principal importance for organizing cortical activity. Furthermore, the PFC E/I balance has been implicated in successful execution of multiple PFC-dependent behaviors in both animal research and the context of human psychiatric disorders.
Methods: Here, we used a pharmacogenetic strategy to decrease mediodorsal thalamic activity in adult male rats and evaluated the consequences for E/I balance in PFC pyramidal neurons as well as cognition, social interaction, and anxiety.
Results: We found that dampening mediodorsal thalamic activity caused significant reductions in gamma-aminobutyric acidergic signaling and increased E/I balance in the mPFC and was concomitant with abnormalities in these behaviors. Furthermore, by selectively activating parvalbumin interneurons in the mPFC with a novel pharmacogenetic approach, we restored gamma-aminobutyric acidergic signaling and E/I balance as well as ameliorated all behavioral impairments.
Conclusions: These findings underscore the importance of thalamocortical activation of mPFC gamma-aminobutyric acidergic interneurons in a broad range of mPFC-dependent behaviors. Furthermore, they highlight this circuitry as a platform for therapeutic investigation in psychiatric diseases that involve impairments in PFC-dependent behaviors.
Keywords: Cognition; GABAergic inhibition; PVIs; Pharmacogenetics; Prefrontal cortex; Synaptic function; Thalamus.
Copyright © 2017 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
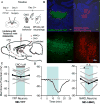
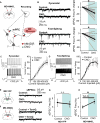

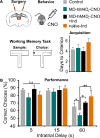


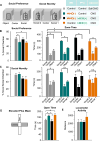
Comment in
-
Medial Prefrontal Cortex Excitation/Inhibition Balance and Schizophrenia-like Behaviors Regulated by Thalamic Inputs to Interneurons.Biol Psychiatry. 2018 Apr 15;83(8):630-631. doi: 10.1016/j.biopsych.2018.02.005. Biol Psychiatry. 2018. PMID: 29559093 No abstract available.
References
-
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

