Effect of Cyclic Dynamic Compressive Loading on Chondrocytes and Adipose-Derived Stem Cells Co-Cultured in Highly Elastic Cryogel Scaffolds
- PMID: 29373507
- PMCID: PMC5855592
- DOI: 10.3390/ijms19020370
Effect of Cyclic Dynamic Compressive Loading on Chondrocytes and Adipose-Derived Stem Cells Co-Cultured in Highly Elastic Cryogel Scaffolds
Abstract
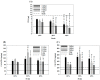
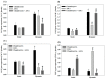
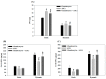
In this study, we first used gelatin/chondroitin-6-sulfate/hyaluronan/chitosan highly elastic cryogels, which showed total recovery from large strains during repeated compression cycles, as 3D scaffolds to study the effects of cyclic dynamic compressive loading on chondrocyte gene expression and extracellular matrix (ECM) production. Dynamic culture of porcine chondrocytes was studied at 1 Hz, 10% to 40% strain and 1 to 9 h/day stimulation duration, in a mechanical-driven multi-chamber bioreactor for 14 days. From the experimental results, we could identify the optimum dynamic culture condition (20% and 3 h/day) to enhance the chondrocytic phenotype of chondrocytes from the expression of marker (Col I, Col II, Col X, TNF-α, TGF-β1 and IGF-1) genes by quantitative real-time polymerase chain reactions (qRT-PCR) and production of ECM (GAGs and Col II) by biochemical analysis and immunofluorescence staining. With up-regulated growth factor (TGF-β1 and IGF-1) genes, co-culture of chondrocytes with porcine adipose-derived stem cells (ASCs) was employed to facilitate chondrogenic differentiation of ASCs during dynamic culture in cryogel scaffolds. By replacing half of the chondrocytes with ASCs during co-culture, we could obtain similar production of ECM (GAGs and Col II) and expression of Col II, but reduced expression of Col I, Col X and TNF-α. Subcutaneous implantation of cells/scaffold constructs in nude mice after mono-culture (chondrocytes or ASCs) or co-culture (chondrocytes + ASCs) and subject to static or dynamic culture condition in vitro for 14 days was tested for tissue-engineering applications. The constructs were retrieved 8 weeks post-implantation for histological analysis by Alcian blue, Safranin O and Col II immunohistochemical staining. The most abundant ectopic cartilage tissue was found for the chondrocytes and chondrocytes + ASCs groups using dynamic culture, which showed similar neo-cartilage formation capability with half of the chondrocytes replaced by ASCs for co-culture. This combined co-culture/dynamic culture strategy is expected to cut down the amount of donor chondrocytes needed for cartilage-tissue engineering.
Keywords: adipose-derived stem cells; cartilage-tissue engineering; chondrocytes; compressive loading; cryogel; dynamic culture; mechanical stimulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Dynamic compression of rabbit adipose-derived stem cells transfected with insulin-like growth factor 1 in chitosan/gelatin scaffolds induces chondrogenesis and matrix biosynthesis.J Cell Physiol. 2012 May;227(5):2003-12. doi: 10.1002/jcp.22927. J Cell Physiol. 2012. PMID: 21751209
-
Chondroitin sulfate/hyaluronic acid/carboxymethylcellulose macroporous cryogels for controlled delivery of TGF-β1 and IGF-1 to induce chondrogenic differentiation of adipose-derived stem cells in cartilage tissue engineering.Int J Biol Macromol. 2025 Jun;317(Pt 2):144756. doi: 10.1016/j.ijbiomac.2025.144756. Epub 2025 May 29. Int J Biol Macromol. 2025. PMID: 40449777
-
Dual Function of Glucosamine in Gelatin/Hyaluronic Acid Cryogel to Modulate Scaffold Mechanical Properties and to Maintain Chondrogenic Phenotype for Cartilage Tissue Engineering.Int J Mol Sci. 2016 Nov 23;17(11):1957. doi: 10.3390/ijms17111957. Int J Mol Sci. 2016. PMID: 27886065 Free PMC article.
-
Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspectives from stem cell biology and molecular medicine.J Cell Physiol. 2013 May;228(5):938-44. doi: 10.1002/jcp.24255. J Cell Physiol. 2013. PMID: 23042088 Review.
-
Co-culture and Mechanical Stimulation on Mesenchymal Stem Cells and Chondrocytes for Cartilage Tissue Engineering.Curr Stem Cell Res Ther. 2020;15(1):54-60. doi: 10.2174/1574888X14666191029104249. Curr Stem Cell Res Ther. 2020. PMID: 31660820 Review.
Cited by
-
Glycosaminoglycan-Based Cryogels as Scaffolds for Cell Cultivation and Tissue Regeneration.Molecules. 2021 Sep 15;26(18):5597. doi: 10.3390/molecules26185597. Molecules. 2021. PMID: 34577067 Free PMC article. Review.
-
Adipose Stem Cell Translational Applications: From Bench-to-Bedside.Int J Mol Sci. 2018 Nov 5;19(11):3475. doi: 10.3390/ijms19113475. Int J Mol Sci. 2018. PMID: 30400641 Free PMC article. Review.
-
Advancements in hydrogel design for articular cartilage regeneration: A comprehensive review.Bioact Mater. 2024 Sep 14;43:1-31. doi: 10.1016/j.bioactmat.2024.09.005. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39318636 Free PMC article. Review.
-
Macroporous Cell-Laden Gelatin/Hyaluronic Acid/Chondroitin Sulfate Cryogels for Engineered Tissue Constructs.Gels. 2022 Sep 16;8(9):590. doi: 10.3390/gels8090590. Gels. 2022. PMID: 36135302 Free PMC article.
-
[Research progress of different mechanical stimulation regulating chondrocytes metabolism].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020 Dec 25;37(6):1101-1108. doi: 10.7507/1001-5515.202001044. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2020. PMID: 33369351 Free PMC article. Review. Chinese.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

