The Ever-Evolving Concept of the Cancer Stem Cell in Pancreatic Cancer
- PMID: 29373514
- PMCID: PMC5836065
- DOI: 10.3390/cancers10020033
The Ever-Evolving Concept of the Cancer Stem Cell in Pancreatic Cancer
Abstract
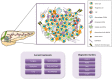
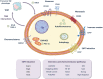
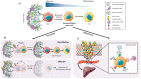
Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer, is the 4th most frequent cause of cancer-related death worldwide, primarily due to the inherent chemoresistant nature and metastatic capacity of this tumor. The latter is believed to be mainly due to the existence of a subpopulation of highly plastic "stem"-like cells within the tumor, known as cancer stem cells (CSCs), which have been shown to have unique metabolic, autophagic, invasive, and chemoresistance properties that allow them to continuously self-renew and escape chemo-therapeutic elimination. As such, current treatments for the majority of PDAC patients are not effective and do not significantly impact overall patient survival (<7 months) as they do not affect the pancreatic CSC (PaCSC) population. In this context, it is important to highlight the need to better understand the characteristics of the PaCSC population in order to develop new therapies to target these cells. In this review, we will provide the latest updates and knowledge on the inherent characteristics of PaCSCs, particularly their unique biological properties including chemoresistance, epithelial to mesenchymal transition, plasticity, metabolism and autophagy.
Keywords: EMT; autophagy; cancer stem cells; chemoresistance; hybrid cancer cell; metabolism; metastasis; pancreatic cancer; plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Scarà S., Bottoni P., Scatena R. CA 19-9: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015;867:247–260. - PubMed
-
- Haab B.B., Huang Y., Balasenthil S., Partyka K., Tang H., Anderson M., Allen P., Sasson A., Zeh H., Kaul K., et al. Definitive Characterization of CA 19-9 in Resectable Pancreatic Cancer Using a Reference Set of Serum and Plasma Specimens. PLoS ONE. 2015;10:e0139049. doi: 10.1371/journal.pone.0139049. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

