Modulation of HIV replication in monocyte derived macrophages (MDM) by steroid hormones
- PMID: 29373606
- PMCID: PMC5786332
- DOI: 10.1371/journal.pone.0191916
Modulation of HIV replication in monocyte derived macrophages (MDM) by steroid hormones
Abstract
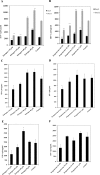
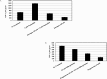
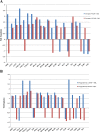
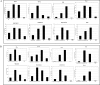
Significant sex specific differences in the progression of HIV/AIDS have been reported. Several studies have implicated steroid hormones in regulating host factor expression and modulating HIV transmission and replication. However, the exact mechanism exerted by steroid hormones estrogen and progesterone in the regulation of HIV-1 replication is still unclear. Results from the current study indicated a dose dependent down regulation of HIV-1 replication in monocyte derived macrophages pre-treated with high concentrations of estrogen or progesterone. To elucidate the molecular mechanisms associated with the down regulation of HIV-1 replication by estrogen and progesterone we used PCR arrays to analyze the expression profile of host genes involved in antiviral responses. Several chemokines, cytokines, transcription factors, interferon stimulated genes and genes involved in type-1 interferon signaling were down regulated in cells infected with HIV-1 pre-treated with high concentrations of estrogen or progesterone compared to untreated HIV-1 infected cells or HIV-1 infected cells treated with low concentrations of estrogen or progesterone. The down regulation of CXCL9, CXCL10 and CXCL11 chemokines and IL-1β, IL-6 cytokines in response to high concentrations of estrogen and progesterone pre-treatment in HIV-1 infected cells was confirmed at the protein level by quantitating chemokine and cytokine concentrations in the culture supernatant. These results demonstrate that a potent anti-inflammatory response is mediated by pre-treatment with high concentrations of estrogen and progesterone. Thus, our study suggests a strong correlation between the down-modulation of anti-viral and pro-inflammatory responses mediated by estrogen and progesterone pre-treatment and the down regulation of HIV-1 replication. These findings may be relevant to clinical observations of sex specific differences in patient populations and point to the need for further investigation.
Conflict of interest statement
Figures

References
-
- Long EM, Martin HL Jr., Kreiss JK, Rainwater SM, Lavreys L, Jackson DJ, et al. Gender differences in HIV-1 diversity at time of infection. Nat Med. 2000;6(1):71–5. Epub 1999/12/29. doi: 10.1038/71563 . - DOI - PubMed
-
- Anastos K, Gange SJ, Lau B, Weiser B, Detels R, Giorgi JV, et al. Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr. 2000;24(3):218–26. Epub 2000/09/02. . - PubMed
-
- Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35(3):313–22. Epub 2002/07/13. doi: CID011586 [pii] doi: 10.1086/341249 . - DOI - PubMed
-
- Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24(6):627–38. . - PubMed
-
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–44. doi: 10.1038/nri2394 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

