Fear Extinction Recall Modulates Human Frontomedial Theta and Amygdala Activity
- PMID: 29373635
- PMCID: PMC6659170
- DOI: 10.1093/cercor/bhx353
Fear Extinction Recall Modulates Human Frontomedial Theta and Amygdala Activity
Abstract
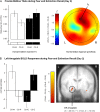
Human functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) studies, as well as animal studies, indicate that the amygdala and frontomedial brain regions are critically involved in conditioned fear and that frontomedial oscillations in the theta range (4-8 Hz) may support communication between these brain regions. However, few studies have used a multimodal approach to probe interactions among these key regions in humans. Here, our goal was to bridge the gap between prior human fMRI, EEG, and animal findings. Using simultaneous EEG-fMRI recordings 24 h after fear conditioning and extinction, conditioned stimuli presented (CS+E, CS-E) and not presented during extinction (CS+N, CS-N) were compared to identify effects specific to extinction versus fear recall. Differential (CS+ vs. CS-) electrodermal, frontomedial theta (EEG) and amygdala responses (fMRI) were reduced for extinguished versus nonextinguished stimuli. Importantly, effects on theta power covaried with effects on amygdala activation. Fear and extinction recall as indicated by theta explained 60% of the variance for the analogous effect in the right amygdala. Our findings show for the first time the interplay of amygdala and frontomedial theta activity during fear and extinction recall in humans and provide insight into neural circuits consistently linked with top-down amygdala modulation in rodents.
Figures



References
-
- Allen PJ, Josephs O, Turner R. 2000. A method for removing imaging artifact from continuous EEG recorded during functional MRI. NeuroImage. 12:230–239. - PubMed
-
- Allen PJ, Polizzi G, Krakow K, Fish DR, Lemieux L. 1998. Identification of EEG events in the MR scanner: the problem of pulse artifact and a method for its subtraction. NeuroImage. 8:229–239. - PubMed
-
- Armony JL, Dolan RJ. 2001. Modulation of auditory neural responses by a visual context in human fear conditioning. Neuroreport. 12:3407–3411. - PubMed
-
- Bastiaansen M, Mazaheri A, Jesen O. 2012. Beyond ERPs: oscillatory neuronal dynamics In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. New York, NY: Oxford University Press. p. 31–49.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

