Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies
- PMID: 29373757
- PMCID: PMC6159538
- DOI: 10.1093/hmg/ddy037
Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies
Abstract
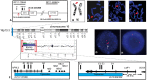
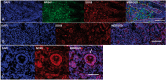
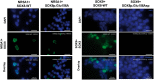
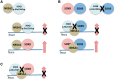
SOX8 is an HMG-box transcription factor closely related to SRY and SOX9. Deletion of the gene encoding Sox8 in mice causes reproductive dysfunction but the role of SOX8 in humans is unknown. Here, we show that SOX8 is expressed in the somatic cells of the early developing gonad in the human and influences human sex determination. We identified two individuals with 46, XY disorders/differences in sex development (DSD) and chromosomal rearrangements encompassing the SOX8 locus and a third individual with 46, XY DSD and a missense mutation in the HMG-box of SOX8. In vitro functional assays indicate that this mutation alters the biological activity of the protein. As an emerging body of evidence suggests that DSDs and infertility can have common etiologies, we also analysed SOX8 in a cohort of infertile men (n = 274) and two independent cohorts of women with primary ovarian insufficiency (POI; n = 153 and n = 104). SOX8 mutations were found at increased frequency in oligozoospermic men (3.5%; P < 0.05) and POI (5.06%; P = 4.5 × 10-5) as compared with fertile/normospermic control populations (0.74%). The mutant proteins identified altered SOX8 biological activity as compared with the wild-type protein. These data demonstrate that SOX8 plays an important role in human reproduction and SOX8 mutations contribute to a spectrum of phenotypes including 46, XY DSD, male infertility and 46, XX POI.
Figures



References
-
- Sekido R., Lovell-Badge R. (2008) Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature, 453, 930–934. - PubMed
-
- Chaboissier M.C., Kobayashi A., Vidal V.I., Lützkendorf S., van de Kant H.J., Wegner M., de Rooij D.G., Behringer R.R., Schedl A. (2004) Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development, 131, 1891–1901. - PubMed
-
- Barrionuevo F., Georg I., Scherthan H., Lécureuil C., Guillou F., Wegner M., Scherer G. (2009) Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev. Biol., 327, 301–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

