Genome Analysis of Fimbriiglobus ruber SP5T, a Planctomycete with Confirmed Chitinolytic Capability
- PMID: 29374042
- PMCID: PMC5861812
- DOI: 10.1128/AEM.02645-17
Genome Analysis of Fimbriiglobus ruber SP5T, a Planctomycete with Confirmed Chitinolytic Capability
Abstract
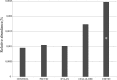
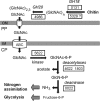
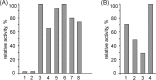
Members of the bacterial order Planctomycetales have often been observed in associations with Crustacea. The ability to degrade chitin, however, has never been reported for any of the cultured planctomycetes although utilization of N-acetylglucosamine (GlcNAc) as a sole carbon and nitrogen source is well recognized for these bacteria. Here, we demonstrate the chitinolytic capability of a member of the family Gemmataceae, Fimbriiglobus ruber SP5T, which was isolated from a peat bog. As revealed by metatranscriptomic analysis of chitin-amended peat, the pool of 16S rRNA reads from F. ruber increased in response to chitin availability. Strain SP5T displayed only weak growth on amorphous chitin as a sole source of carbon but grew well with chitin as a source of nitrogen. The genome of F. ruber SP5T is 12.364 Mb in size and is the largest among all currently determined planctomycete genomes. It encodes several enzymes putatively involved in chitin degradation, including two chitinases affiliated with the glycoside hydrolase (GH) family GH18, GH20 family β-N-acetylglucosaminidase, and the complete set of enzymes required for utilization of GlcNAc. The gene encoding one of the predicted chitinases was expressed in Escherichia coli, and the endochitinase activity of the recombinant enzyme was confirmed. The genome also contains genes required for the assembly of type IV pili, which may be used to adhere to chitin and possibly other biopolymers. The ability to use chitin as a source of nitrogen is of special importance for planctomycetes that inhabit N-depleted ombrotrophic wetlands.IMPORTANCE Planctomycetes represent an important part of the microbial community in Sphagnum-dominated peatlands, but their potential functions in these ecosystems remain poorly understood. This study reports the presence of chitinolytic potential in one of the recently described peat-inhabiting members of the family Gemmataceae, Fimbriiglobus ruber SP5T This planctomycete uses chitin, a major constituent of fungal cell walls and exoskeletons of peat-inhabiting arthropods, as a source of nitrogen in N-depleted ombrotrophic Sphagnum-dominated peatlands. This study reports the chitin-degrading capability of representatives of the order Planctomycetales.
Keywords: Fimbriiglobus ruber; Gemmataceae; Planctomycetes; chitinase; chitinolytic ability; genome annotation.
Copyright © 2018 American Society for Microbiology.
Figures

References
-
- Ward NL. 2010. Phylum XXV. Planctomycetes Garrity and Holt 2001, 137 emend. Ward, p 879–925. In Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB (ed), Bergey's manual of systematic bacteriology, vol 4 Springer, New York, NY.
-
- Fukunaga Y, Kurahashi M, Sakiyama Y, Ohuchi M, Yokota A, Harayama S. 2009. Phycisphaera mikurensis gen. nov., sp. nov., isolated from a marine alga, and proposal of Phycisphaeraceae fam. nov., Phycisphaerales ord. nov. and Phycisphaerae classis nov. in the phylum Planctomycetes. J Gen Appl Microbiol 55:267–275. doi:10.2323/jgam.55.267. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

