PAD4 Deficiency Leads to Decreased Organ Dysfunction and Improved Survival in a Dual Insult Model of Hemorrhagic Shock and Sepsis
- PMID: 29374076
- PMCID: PMC5821587
- DOI: 10.4049/jimmunol.1700639
PAD4 Deficiency Leads to Decreased Organ Dysfunction and Improved Survival in a Dual Insult Model of Hemorrhagic Shock and Sepsis
Abstract
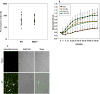
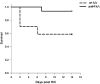
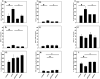
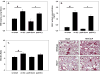
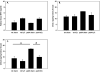
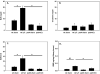
Indirect acute respiratory distress syndrome (iARDS) is caused by a nonpulmonary inflammatory process resulting from insults such as nonpulmonary sepsis. Neutrophils are thought to play a significant role in mediating ARDS, with the development of iARDS being characterized by dysregulation and recruitment of activated neutrophils into the lung. Recently, a novel mechanism of microbial killing by neutrophils was identified through the formation of neutrophil extracellular traps (NETs). NETs are composed of large webs of decondensed chromatin released from activated neutrophils into the extracellular space; they are regulated by the enzyme peptidylarginine deiminase 4 (PAD4) through mediation of chromatin decondensation via citrullination of target histones. Components of NETs have been implicated in ARDS. However, it is unknown whether there is any pathological significance of NET formation in ARDS caused indirectly by nonpulmonary insult. We subjected PAD4-/- mice and wild-type mice to a "two-hit" model of hypovolemic shock (fixed-pressure hemorrhage [Hem]) followed by septic cecal ligation and puncture (CLP) insult (Hem/CLP). Mice were hemorrhaged and resuscitated; 24 h after Hem, mice were then subjected to CLP. Overall, PAD4 deletion led to an improved survival as compared with wild-type mice. PAD4-/- mice displayed a marked decrease in neutrophil influx into the lung, as well decreased presence of proinflammatory mediators. PAD4-/- mice were also able to maintain baseline kidney function after Hem/CLP. These data taken together suggest PAD4-mediated NET formation contributes to the mortality associated with shock/sepsis and may play a role in the pathobiology of end organ injury in response to combined hemorrhage plus sepsis.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures



References
-
- Lomas-Neira J, Chung CS, Perl M, Gregory S, Biffl W, Ayala A. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Am J Physiol Lung Cell Mol Physiol. 2006;290:L51–8. - PubMed
-
- Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–64. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

