Subcortical evidence for a contribution of arousal to fMRI studies of brain activity
- PMID: 29374172
- PMCID: PMC5786066
- DOI: 10.1038/s41467-017-02815-3
Subcortical evidence for a contribution of arousal to fMRI studies of brain activity
Abstract
Cortical activity during periods of rest is punctuated by widespread, synchronous events in both electrophysiological and hemodynamic signals, but their behavioral relevance remains unclear. Here we report that these events correspond to momentary drops in cortical arousal and are associated with activity changes in the basal forebrain and thalamus. Combining fMRI and electrophysiology in macaques, we first establish that fMRI transients co-occur with spectral shifts in local field potentials (LFPs) toward low frequencies. Applying this knowledge to fMRI data from the human connectome project, we find that the fMRI transients are strongest in sensory cortices. Surprisingly, the positive cortical transients occur together with negative transients in focal subcortical areas known to be involved with arousal regulation, most notably the basal forebrain. This subcortical involvement, combined with the prototypical pattern of LFP spectral shifts, suggests that commonly observed widespread variations in fMRI cortical activity are associated with momentary drops in arousal.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

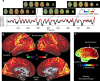

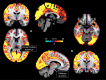

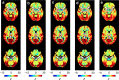
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

