New role of phenothiazine derivatives as peripherally acting CB1 receptor antagonizing anti-obesity agents
- PMID: 29374224
- PMCID: PMC5785958
- DOI: 10.1038/s41598-018-20078-w
New role of phenothiazine derivatives as peripherally acting CB1 receptor antagonizing anti-obesity agents
Abstract
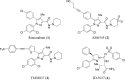
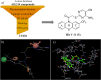
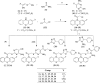
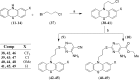
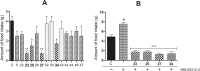
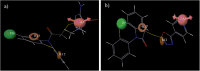
Developing peripherally active cannabinoid 1 (CB1) receptor antagonists is a novel therapeutic approach for the management of obesity. An unusual phenothiazine scaffold containing CB1R antagonizing hit was identified by adopting virtual screening work flow. The hit so identified was further modified by introducing polar functional groups into it to enhance the polar surface area and decrease the hydrophobicity of the resulting molecules. CB1 receptor antagonistic activity for the designed compounds was computed by the previously established pharmacophore and three dimensional quantitative structure-activity relationship models. Docking studies of these designed compounds confirmed the existence of favourable interactions within the active site of the CB1 receptor. The designed compounds were synthesized and evaluated for their CB1 receptor antagonistic activity. Parallel artificial membrane permeability assay was performed to evaluate their potential to permeate into the central nervous system wherein it was observed that the compounds did not possess the propensity to cross the blood brain barrier and would be devoid of central nervous system side effects. In pharmacological evaluation, the synthesized compounds (23, 25, 27 and 34) showed significant decrease in food intake suggesting their potential application in the management of obesity through CB1 receptor antagonist activity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Prospective therapeutic agents for obesity: molecular modification approaches of centrally and peripherally acting selective cannabinoid 1 receptor antagonists.Eur J Med Chem. 2014 May 22;79:298-339. doi: 10.1016/j.ejmech.2014.04.011. Epub 2014 Apr 5. Eur J Med Chem. 2014. PMID: 24747288 Review.
-
The effect of am 251, a cannabinoid CB1 receptor antagonist, on food intake in rats.Acta Pol Pharm. 2004 Sep-Oct;61(5):401-3. Acta Pol Pharm. 2004. PMID: 15747698
-
Pharmacological comparison of traditional and non-traditional cannabinoid receptor 1 blockers in rodent models in vivo.Pharmacol Biochem Behav. 2017 Aug;159:24-35. doi: 10.1016/j.pbb.2017.06.012. Epub 2017 Jun 27. Pharmacol Biochem Behav. 2017. PMID: 28666894
-
Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1H-1,2,4-triazole--LH 21.Neuropharmacology. 2006 Aug;51(2):358-66. doi: 10.1016/j.neuropharm.2006.03.029. Epub 2006 Jun 5. Neuropharmacology. 2006. PMID: 16750544
-
A comprehensive patents review on cannabinoid 1 receptor antagonists as antiobesity agents.Expert Opin Ther Pat. 2015;25(10):1093-116. doi: 10.1517/13543776.2015.1064898. Epub 2015 Jul 10. Expert Opin Ther Pat. 2015. PMID: 26161824 Review.
Cited by
-
Pharmacophore-based virtual screening from phytocannabinoids as antagonist r-CB1.J Mol Model. 2022 Aug 17;28(9):258. doi: 10.1007/s00894-022-05219-3. J Mol Model. 2022. PMID: 35978141
-
The Cannabinoid CB1 Antagonist TM38837 With Limited Penetrance to the Brain Shows Reduced Fear-Promoting Effects in Mice.Front Pharmacol. 2019 Mar 20;10:207. doi: 10.3389/fphar.2019.00207. eCollection 2019. Front Pharmacol. 2019. PMID: 30949045 Free PMC article.
-
Development of Phenothiazine Hybrids with Potential Medicinal Interest: A Review.Molecules. 2022 Jan 3;27(1):276. doi: 10.3390/molecules27010276. Molecules. 2022. PMID: 35011508 Free PMC article. Review.
-
Multiple endocannabinoid-mediated mechanisms in the regulation of energy homeostasis in brain and peripheral tissues.Cell Mol Life Sci. 2019 Apr;76(7):1341-1363. doi: 10.1007/s00018-018-2994-6. Epub 2019 Jan 1. Cell Mol Life Sci. 2019. PMID: 30599065 Free PMC article. Review.
-
Discovery of Natural Product Derived Labdane Appended Triazoles as Potent Pancreatic Lipase Inhibitors.ACS Med Chem Lett. 2018 Jun 18;9(7):662-666. doi: 10.1021/acsmedchemlett.8b00109. eCollection 2018 Jul 12. ACS Med Chem Lett. 2018. PMID: 30034597 Free PMC article.
References
-
- World Health Organization. Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/ (2017).
-
- Sharma MK, Murumkar PR, Kanhed AM, Giridhar R, Yadav MR. Prospective therapeutic agents for obesity: molecular modification approaches of centrally and peripherally acting selective cannabinoid 1 receptor antagonists. Eur. J. Med. Chem. 2014;79:298–339. doi: 10.1016/j.ejmech.2014.04.011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

