Non-parenchymal TREM-2 protects the liver from immune-mediated hepatocellular damage
- PMID: 29374630
- PMCID: PMC6580759
- DOI: 10.1136/gutjnl-2017-314107
Non-parenchymal TREM-2 protects the liver from immune-mediated hepatocellular damage
Abstract
Objective: Liver injury impacts hepatic inflammation in part via Toll-like receptor (TLR) signalling. Triggering receptor expressed on myeloid cells 2 (TREM-2) modulates TLR4-mediated inflammation in bone marrow (BM)-derived macrophages but its function in liver injury is unknown. Here we hypothesised that the anti-inflammatory effects of TREM-2 on TLR signalling may limit hepatic injury.
Design: TREM-2 expression was analysed in livers of humans with various forms of liver injury compared with control individuals. Acute and chronic liver injury models were performed in wild type and Trem-2-/- mice. Primary liver cells from both genotypes of mice were isolated for in vitro experiments.
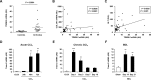
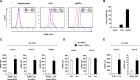
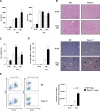
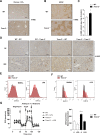
Results: TREM-2 was expressed on non-parenchymal hepatic cells and induced during liver injury in mice and man. Mice lacking TREM-2 exhibited heightened liver damage and inflammation during acute and repetitive carbon tetrachloride and acetaminophen (APAP) intoxication, the latter of which TREM-2 deficiency was remarkably associated with worsened survival. Liver damage in Trem-2-/- mice following chronic injury and APAP challenge was associated with elevated hepatic lipid peroxidation and macrophage content. BM transplantation experiments and cellular reactive oxygen species assays revealed effects of TREM-2 in the context of chronic injury depended on both immune and resident TREM-2 expression. Consistent with effects of TREM-2 on inflammation-associated injury, primary hepatic macrophages and hepatic stellate cells lacking TREM-2 exhibited augmented TLR4-driven proinflammatory responses.
Conclusion: Our data indicate that by acting as a natural brake on inflammation during hepatocellular injury, TREM-2 is a critical regulator of diverse types of hepatotoxic injury.
Keywords: acute liver failure; chronic liver disease; hepatic stellate cell; immune-mediated liver damage; inflammation.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




Comment in
-
Liver: A protective role for TREM2 in liver injury.Nat Rev Gastroenterol Hepatol. 2018 Mar;15(3):130-131. doi: 10.1038/nrgastro.2018.13. Epub 2018 Feb 14. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29443116 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
