Study protocol for a cluster randomised controlled factorial design trial to assess the effectiveness and feasibility of reactive focal mass drug administration and vector control to reduce malaria transmission in the low endemic setting of Namibia
- PMID: 29374672
- PMCID: PMC5829876
- DOI: 10.1136/bmjopen-2017-019294
Study protocol for a cluster randomised controlled factorial design trial to assess the effectiveness and feasibility of reactive focal mass drug administration and vector control to reduce malaria transmission in the low endemic setting of Namibia
Abstract
Introduction: To interrupt malaria transmission, strategies must target the parasite reservoir in both humans and mosquitos. Testing of community members linked to an index case, termed reactive case detection (RACD), is commonly implemented in low transmission areas, though its impact may be limited by the sensitivity of current diagnostics. Indoor residual spraying (IRS) before malaria season is a cornerstone of vector control efforts. Despite their implementation in Namibia, a country approaching elimination, these methods have been met with recent plateaus in transmission reduction. This study evaluates the effectiveness and feasibility of two new targeted strategies, reactive focal mass drug administration (rfMDA) and reactive focal vector control (RAVC) in Namibia.
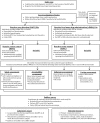
Methods and analysis: This is an open-label cluster randomised controlled trial with 2×2 factorial design. The interventions include: rfMDA (presumptive treatment with artemether-lumefantrine (AL)) versus RACD (rapid diagnostic testing and treatment using AL) and RAVC (IRS with Acellic 300CS) versus no RAVC. Factorial design also enables comparison of the combined rfMDA+RAVC intervention to RACD. Participants living in 56 enumeration areas will be randomised to one of four arms: rfMDA, rfMDA+RAVC, RACD or RACD+RAVC. These interventions, triggered by index cases detected at health facilities, will be targeted to individuals residing within 500 m of an index. The primary outcome is cumulative incidence of locally acquired malaria detected at health facilities over 1 year. Secondary outcomes include seroprevalence, infection prevalence, intervention coverage, safety, acceptability, adherence, cost and cost-effectiveness.
Ethics and dissemination: Findings will be reported on clinicaltrials.gov, in peer-reviewed publications and through stakeholder meetings with MoHSS and community leaders in Namibia.
Trial registration number: NCT02610400; Pre-results.
Keywords: Plasmodium falciparum; Namibia; artemether-lumefantrine; cluster randomisation; elimination; indoor residual spraying; low transmission; malaria; mass drug administration; pirimiphos-methyl; presumptive treatment; reactive case detection; vector control.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: AA and ZG are employed by Novartis Foundation. All other authors declare no competing interests.
Figures
References
-
- Harris I, Sharrock WW, Bain LM, et al. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J 2010;9:254 10.1186/1475-2875-9-254 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous