TEXT messages to improve MEDication adherence and Secondary prevention (TEXTMEDS) after acute coronary syndrome: a randomised clinical trial protocol
- PMID: 29374674
- PMCID: PMC5829769
- DOI: 10.1136/bmjopen-2017-019463
TEXT messages to improve MEDication adherence and Secondary prevention (TEXTMEDS) after acute coronary syndrome: a randomised clinical trial protocol
Abstract
Background: Identifying simple, low-cost and scalable means of supporting lifestyle change and medication adherence for patients following a cardiovascular (CV) event is important.
Objective: The TEXTMEDS (TEXT messages to improve MEDication adherence and Secondary prevention) study aims to investigate whether a cardiac education and support programme sent via mobile phone text message improves medication adherence and risk factor levels in patients following an acute coronary syndrome (ACS).
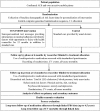
Study design: A single-blind, multicentre, randomised clinical trial of 1400 patients after an ACS with 12 months follow-up. The intervention group will receive multiple weekly text messages that provide information, motivation, support to adhere to medications, quit smoking (if relevant) and recommendations for healthy diet and exercise. The primary endpoint is the percentage of patients who are adherent to cardioprotective medications and the key secondary outcomes are mean systolic blood pressure (BP) and low-density lipoprotein cholesterol. Secondary outcomes will also include total cholesterol, mean diastolic BP, the percentage of participants who are adherent to each cardioprotective medication class, the percentage of participants who achieve target levels of CV risk factors, major vascular events, hospital readmissions and all-cause mortality. The study will be augmented by formal economic and process evaluations to assess acceptability, utility and cost-effectiveness.
Summary: The study will provide multicentre randomised trial evidence of the effects of a text message-based programme on cardioprotective medication adherence and levels of CV risk factors.
Ethics and dissemination: Primary ethics approval was received from Western Sydney Local Health District Human Research Ethics Committee (HREC2012/12/4.1 (3648) AU RED HREC/13/WMEAD/15). Results will be disseminated via peer-reviewed publications and presentations at international conferences.
Trial registration number: ACTRN12613000793718; Pre-results.
Keywords: cardiovascular risk factors; coronary heart disease; medication adherence; mhealth; secondary prevention; text message.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Organisation WH. The top 10 cause of death. Fact Sheet No 310. (accessed may 2014).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
