Chronic Alcohol, Intrinsic Excitability, and Potassium Channels: Neuroadaptations and Drinking Behavior
- PMID: 29374839
- PMCID: PMC8252120
- DOI: 10.1007/164_2017_90
Chronic Alcohol, Intrinsic Excitability, and Potassium Channels: Neuroadaptations and Drinking Behavior
Erratum in
-
Correction to: Chronic Alcohol, Intrinsic Excitability, and Potassium Channels: Neuroadaptations and Drinking Behavior.Handb Exp Pharmacol. 2018;248:619. doi: 10.1007/164_2018_192. Handb Exp Pharmacol. 2018. PMID: 30810861
Abstract
Neural mechanisms underlying alcohol use disorder remain elusive, and this lack of understanding has slowed the development of efficacious treatment strategies for reducing relapse rates and prolonging abstinence. While synaptic adaptations produced by chronic alcohol exposure have been extensively characterized in a variety of brain regions, changes in intrinsic excitability of critical projection neurons are understudied. Accumulating evidence suggests that prolonged alcohol drinking and alcohol dependence produce plasticity of intrinsic excitability as measured by changes in evoked action potential firing and after-hyperpolarization amplitude. In this chapter, we describe functional changes in cell firing of projection neurons after long-term alcohol exposure that occur across species and in multiple brain regions. Adaptations in calcium-activated (KCa2), voltage-dependent (KV7), and G protein-coupled inwardly rectifying (Kir3 or GIRK) potassium channels that regulate the evoked firing and after-hyperpolarization parallel functional changes in intrinsic excitability induced by chronic alcohol. Moreover, there are strong genetic links between alcohol-related behaviors and genes encoding KCa2, KV7, and GIRK channels, and pharmacologically targeting these channels reduces alcohol consumption and alcohol-related behaviors. Together, these studies demonstrate that chronic alcohol drinking produces adaptations in KCa2, KV7, and GIRK channels leading to impaired regulation of the after-hyperpolarization and aberrant cell firing. Correcting the deficit in the after-hyperpolarization with positive modulators of KCa2 and KV7 channels and altering the GIRK channel binding pocket to block the access of alcohol represent a potentially highly effective pharmacological approach that can restore changes in intrinsic excitability and reduce alcohol consumption in affected individuals.
Keywords: After-hyperpolarization; Alcohol drinking; Alcohol use disorder; Intrinsic excitability; Potassium channels.
Figures
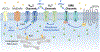

References
-
- DSM-5, Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013. 5th edn.
-
- SAMHSA, Substance Abuse and Mental Health Administration (SAMHSA). 2015 National Survey on Drug Use and Health (NSDUH). 2015.
-
- Kourrich S, Calu DJ, and Bonci A, Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci, 2015. 16(3): p. 173–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

