Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK
- PMID: 29375036
- PMCID: PMC5787923
- DOI: 10.1186/s12916-017-0989-z
Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK
Abstract
Background: Rotavirus causes severe gastroenteritis in infants and young children worldwide. The UK introduced the monovalent rotavirus vaccine (Rotarix®) in July 2013. Vaccination is free of charge to parents, with two doses delivered at 8 and 12 weeks of age. We evaluated vaccine impact across a health system in relation to socioeconomic deprivation.
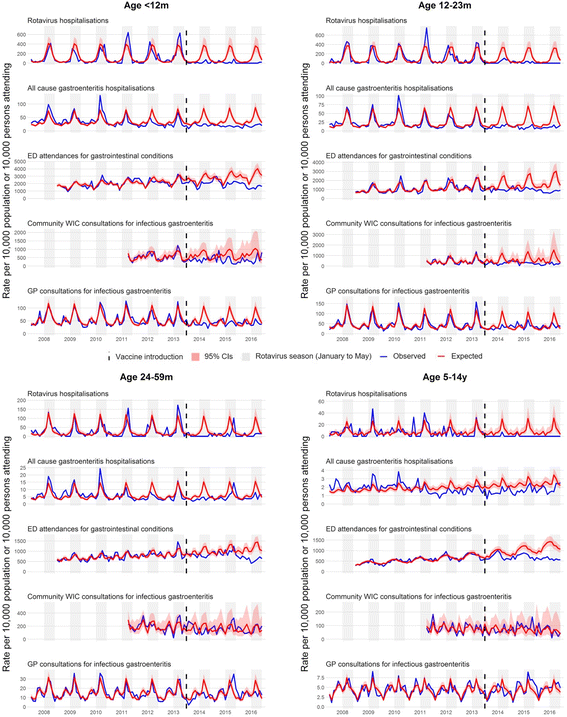
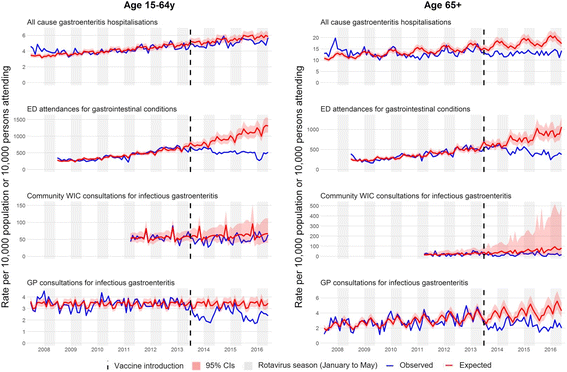
Methods: We used interrupted time-series analyses to assess changes in monthly health-care attendances in Merseyside, UK, for all ages, from July 2013 to June 2016, compared to predicted counterfactual attendances without vaccination spanning 3-11 years pre-vaccine. Outcome measures included laboratory-confirmed rotavirus gastroenteritis (RVGE) hospitalisations, acute gastroenteritis (AGE) hospitalisations, emergency department (ED) attendances for gastrointestinal conditions and consultations for infectious gastroenteritis at community walk-in centres (WIC) and general practices (GP). All analyses were stratified by age. Hospitalisations were additionally stratified by vaccine uptake and small-area-level socioeconomic deprivation.
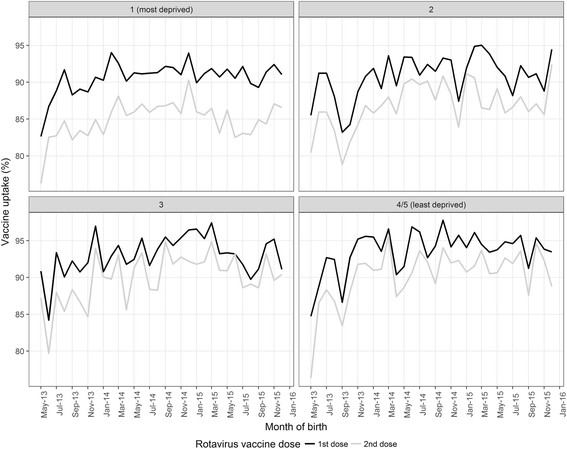
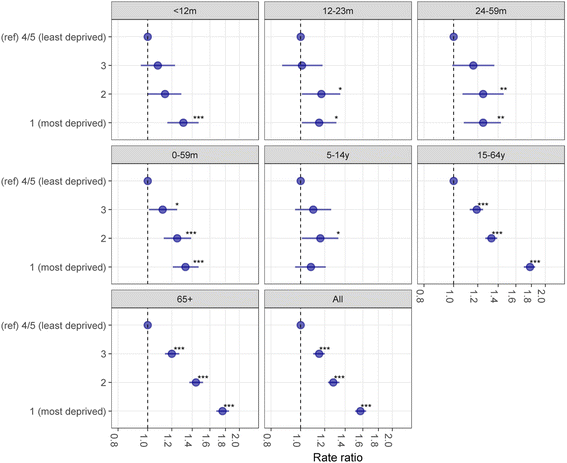
Results: The uptake of the first and second doses of rotavirus vaccine was 91.4% (29,108/31,836) and 86.7% (27,594/31,836), respectively. Among children aged < 5 years, the incidence of gastrointestinal disease decreased across all outcomes post-vaccine introduction: 80% (95% confidence interval [CI] 70-87%; p < 0.001) for RVGE hospitalisation, 44% (95% CI 35-53%; p < 0.001) for AGE hospitalisations, 23% (95% CI 11-33%; p < 0.001) for ED, 32% (95% CI 7-50%; p = 0.02) for WIC and 13% (95% CI -3-26%; p = 0.10) for GP. The impact was greatest during the rotavirus season and for vaccine-eligible age groups. In adults aged 65+ years, AGE hospitalisations fell by 25% (95% CI 19-30%; p < 0.001). The pre-vaccine risk of AGE hospitalisation was highest in the most socioeconomically deprived communities (adjusted incident rate ratio 1.57; 95% CI 1.51-1.64; p < 0.001), as was the risk for non-vaccination (adjusted risk ratio 1.54; 95% CI 1.34-1.75; p < 0.001). The rate of AGE hospitalisations averted per 1,000 first doses of vaccine was higher among infants in the most deprived communities compared to the least deprived in 2014/15 (28; 95% CI 25-31 vs. 15; 95% CI 12-17) and in 2015/16 (26; 95% CI 23-30 vs. 13; 95% CI 11-16).
Conclusions: Following the introduction of rotavirus vaccination, incidence of gastrointestinal disease reduced across the health-care system. Vaccine impact was greatest among the most deprived populations, despite lower vaccine uptake. Prioritising vaccine uptake in socioeconomically deprived communities should give the greatest health benefit in terms of population disease burden.
Keywords: Diarrhoea; Epidemiology; Gastroenteritis; Health equity; Health service; Paediatric; Rotavirus; Socioeconomic inequalities; Surveillance; Vaccine.
Conflict of interest statement
Consent for publication
Not applicable
Competing interests
All authors have completed the ICMJE uniform disclosure form at
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. - DOI - PubMed
-
- World Health Organization Global networks of rotavirus gastroenteritis, 2001–2008. Wkly Epidemiol Rec. 2008;83:421–8. - PubMed
-
- ROTA Council. Rotavirus deaths & rotavirus vaccine introduction maps – ROTA Council. Global Introduction Status. http://rotacouncil.org/vaccine-introduction/global-introduction-status/. Accessed 16 Nov 2016.
-
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization–coordinated global rotavirus surveillance network Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96–105. doi: 10.1093/cid/civ1013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

