Neuronal Migration and Lamination in the Vertebrate Retina
- PMID: 29375289
- PMCID: PMC5767219
- DOI: 10.3389/fnins.2017.00742
Neuronal Migration and Lamination in the Vertebrate Retina
Abstract
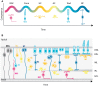
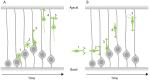
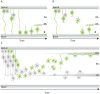
In the retina, like in most other brain regions, developing neurons are arranged into distinct layers giving the mature tissue its stratified appearance. This process needs to be highly controlled and orchestrated, as neuronal layering defects lead to impaired retinal function. To achieve successful neuronal layering and lamination in the retina and beyond, three main developmental steps need to be executed: First, the correct type of neuron has to be generated at a precise developmental time. Second, as most retinal neurons are born away from the position at which they later function, newborn neurons have to move to their final layer within the developing tissue, a process also termed neuronal lamination. Third, these neurons need to connect to their correct synaptic partners. Here, we discuss neuronal migration and lamination in the vertebrate retina and summarize our knowledge on these aspects of retinal development. We give an overview of how lamination emerges and discuss the different modes of neuronal translocation that occur during retinogenesis and what we know about the cell biological machineries driving them. In addition, retinal mosaics and their importance for correct retinal function are examined. We close by stating the open questions and future directions in this exciting field.
Keywords: connectivity; lamination; mosaics; neuronal migration; retina.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

