Fear Learning Regulates Cortical Sensory Representations by Suppressing Habituation
- PMID: 29375323
- PMCID: PMC5767681
- DOI: 10.3389/fncir.2017.00112
Fear Learning Regulates Cortical Sensory Representations by Suppressing Habituation
Abstract
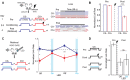
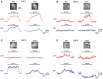
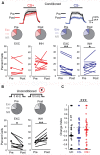
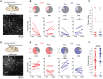
Projections from auditory cortex to the amygdala are thought to contribute to the induction of auditory fear learning. In addition, fear conditioning has been found to enhance cortical responses to conditioned tones, suggesting that cortical plasticity contributes to fear learning. However, the functional role of auditory cortex in the retrieval of fear memories is unclear and how fear learning regulates cortical sensory representations is not well understood. To address these questions, we use acute optogenetic silencing and chronic two-photon calcium imaging in mouse auditory cortex during fear learning. Longitudinal imaging of neuronal ensemble activity reveals that discriminative fear learning modulates cortical sensory representations via the suppression of cortical habituation.
Keywords: GCaMP; auditory cortex; cortical circuits; fear conditioning; interneurons; learning and memory; somatostatin.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

