Bovine Lactoferrin and Lactoferrin-Derived Peptides Inhibit the Growth of Vibrio cholerae and Other Vibrio species
- PMID: 29375503
- PMCID: PMC5768654
- DOI: 10.3389/fmicb.2017.02633
Bovine Lactoferrin and Lactoferrin-Derived Peptides Inhibit the Growth of Vibrio cholerae and Other Vibrio species
Abstract
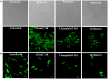
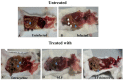
Vibrio is a genus of Gram-negative bacteria, some of which can cause serious infectious diseases. Vibrio infections are associated with the consumption of contaminated food and classified in Vibrio cholera infections and non-cholera Vibrio infections. In the present study, we investigate whether bovine lactoferrin (bLF) and several synthetic peptides corresponding to bLF sequences, are able to inhibit the growth or have bactericidal effect against V. cholerae and other Vibrio species. The antibacterial activity of LF and LF-peptides was assessed by kinetics of growth or determination of colony forming unit in bacteria treated with the peptides and antibiotics. To get insight in the mode of action, the interaction between bLF and bLF-peptides (coupled to FITC) and V. cholera was evaluated. The damage of effector-induced bacterial membrane permeability was measured by inclusion of the fluorescent dye propidium iodide using flow cytometry, whereas the bacterial ultrastructural damage in bacteria treated was observed by transmission electron microscopy. The results showed that bLF and LFchimera inhibited the growth of the V. cholerae strains; LFchimera permeabilized the bacteria which membranes were seriously damaged. Assays with a multidrug-resistant strain of Vibrio species indicated that combination of sub-lethal doses of LFchimera with ampicillin or tetracycline strongly reduced the concentration of the antibiotics to reach 95% growth inhibition. Furthermore, LFchimera were effective to inhibit the V. cholerae counts and damage due to this bacterium in a model mice. These data suggest that LFchimera and bLF are potential candidates to combat the V. cholerae and other multidrug resistant Vibrio species.
Keywords: LFchimera; Vibrio cholerae; bactericide; lactoferrin; lactoferrin peptides.
Figures





Similar articles
-
Bactericidal effect of lactoferrin and lactoferrin chimera against halophilic Vibrio parahaemolyticus.Biochimie. 2009 Jan;91(1):133-40. doi: 10.1016/j.biochi.2008.06.009. Epub 2008 Jun 26. Biochimie. 2009. PMID: 18625283
-
Bactericidal effect of bovine lactoferrin and synthetic peptide lactoferrin chimera in Streptococcus pneumoniae and the decrease in luxS gene expression by lactoferrin.Biometals. 2014 Oct;27(5):969-80. doi: 10.1007/s10534-014-9775-y. Epub 2014 Jul 23. Biometals. 2014. PMID: 25053107
-
Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli.Biometals. 2010 Jun;23(3):569-78. doi: 10.1007/s10534-010-9306-4. Epub 2010 Mar 2. Biometals. 2010. PMID: 20195887
-
Utilization of lactoferrin to fight antibiotic-resistant mammary gland pathogens.J Anim Sci. 2008 Mar;86(13 Suppl):66-71. doi: 10.2527/jas.2007-0216. Epub 2007 Jun 12. J Anim Sci. 2008. PMID: 17565052 Review.
-
Molecular Adaptations and Antibiotic Resistance in Vibrio cholerae: A Communal Challenge.Microb Drug Resist. 2019 Sep;25(7):1012-1022. doi: 10.1089/mdr.2018.0354. Epub 2019 Apr 25. Microb Drug Resist. 2019. PMID: 31021308 Review.
Cited by
-
Bovine Lactoferrin and Hen Ovotransferrin Affect Virulence Factors of Acute Hepatopancreatic Necrosis Disease (AHPND)-Inducing Vibrio parahaemolyticus Strains.Microorganisms. 2023 Dec 2;11(12):2912. doi: 10.3390/microorganisms11122912. Microorganisms. 2023. PMID: 38138056 Free PMC article.
-
Activity of Apo-Lactoferrin on Pathogenic Protozoa.Pharmaceutics. 2022 Aug 15;14(8):1702. doi: 10.3390/pharmaceutics14081702. Pharmaceutics. 2022. PMID: 36015327 Free PMC article. Review.
-
Supplementation of tuna hydrolysate and insect larvae improves fishmeal replacement efficacy of poultry by-product in Lates calcarifer (Bloch, 1790) juveniles.Sci Rep. 2021 Mar 2;11(1):4997. doi: 10.1038/s41598-021-84660-5. Sci Rep. 2021. PMID: 33654188 Free PMC article.
-
The anticancer activity of bovine lactoferrin is reduced by deglycosylation and it follows a different pathway in cervix and colon cancer cells.Food Sci Nutr. 2024 Mar 7;12(5):3516-3528. doi: 10.1002/fsn3.4020. eCollection 2024 May. Food Sci Nutr. 2024. PMID: 38726451 Free PMC article.
-
Lactoferrin and Its Derived Peptides: An Alternative for Combating Virulence Mechanisms Developed by Pathogens.Molecules. 2020 Dec 8;25(24):5763. doi: 10.3390/molecules25245763. Molecules. 2020. PMID: 33302377 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

