Probing the Structures of Viral RNA Regulatory Elements with SHAPE and Related Methodologies
- PMID: 29375504
- PMCID: PMC5767303
- DOI: 10.3389/fmicb.2017.02634
Probing the Structures of Viral RNA Regulatory Elements with SHAPE and Related Methodologies
Abstract
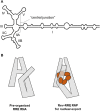
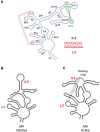
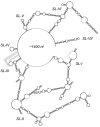
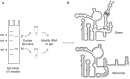
Viral RNAs were selected by evolution to possess maximum functionality in a minimal sequence. Depending on the classification of the virus and the type of RNA in question, viral RNAs must alternately be replicated, spliced, transcribed, transported from the nucleus into the cytoplasm, translated and/or packaged into nascent virions, and in most cases, provide the sequence and structural determinants to facilitate these processes. One consequence of this compact multifunctionality is that viral RNA structures can be exquisitely complex, often involving intermolecular interactions with RNA or protein, intramolecular interactions between sequence segments separated by several thousands of nucleotides, or specialized motifs such as pseudoknots or kissing loops. The fluidity of viral RNA structure can also present a challenge when attempting to characterize it, as genomic RNAs especially are likely to sample numerous conformations at various stages of the virus life cycle. Here we review advances in chemoenzymatic structure probing that have made it possible to address such challenges with respect to cis-acting elements, full-length viral genomes and long non-coding RNAs that play a major role in regulating viral gene expression.
Keywords: RNA structure; SHAPE; chemical probing; secondary structure; viral RNA.
Figures

Similar articles
-
Subcellular Localization of HIV-1 gag-pol mRNAs Regulates Sites of Virion Assembly.J Virol. 2017 Feb 28;91(6):e02315-16. doi: 10.1128/JVI.02315-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053097 Free PMC article.
-
The molecular interactions that stabilize RNA tertiary structure: RNA motifs, patterns, and networks.Acc Chem Res. 2011 Dec 20;44(12):1302-11. doi: 10.1021/ar200098t. Epub 2011 Sep 7. Acc Chem Res. 2011. PMID: 21899297
-
Sequence and structural elements at the 3' terminus of bovine viral diarrhea virus genomic RNA: functional role during RNA replication.J Virol. 1999 May;73(5):3638-48. doi: 10.1128/JVI.73.5.3638-3648.1999. J Virol. 1999. PMID: 10196256 Free PMC article.
-
Orchestrating the Selection and Packaging of Genomic RNA by Retroviruses: An Ensemble of Viral and Host Factors.Viruses. 2016 Sep 20;8(9):257. doi: 10.3390/v8090257. Viruses. 2016. PMID: 27657110 Free PMC article. Review.
-
Know Your Enemy: Successful Bioinformatic Approaches to Predict Functional RNA Structures in Viral RNAs.Front Microbiol. 2018 Jan 4;8:2582. doi: 10.3389/fmicb.2017.02582. eCollection 2017. Front Microbiol. 2018. PMID: 29354101 Free PMC article. Review.
Cited by
-
Application of Proteomics Technology Based on LC-MS Combined with Western Blotting and Co-IP in Antiviral Innate Immunity.Methods Mol Biol. 2025;2854:93-106. doi: 10.1007/978-1-0716-4108-8_11. Methods Mol Biol. 2025. PMID: 39192122
-
Comprehensive in virio structure probing analysis of the influenza A virus identifies functional RNA structures involved in viral genome replication.Comput Struct Biotechnol J. 2023 Oct 19;21:5259-5272. doi: 10.1016/j.csbj.2023.10.036. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37954152 Free PMC article.
-
RNA Structure-A Neglected Puppet Master for the Evolution of Virus and Host Immunity.Front Immunol. 2018 Sep 19;9:2097. doi: 10.3389/fimmu.2018.02097. eCollection 2018. Front Immunol. 2018. PMID: 30283444 Free PMC article. Review.
-
High-resolution RNA tertiary structures in Zika virus stem-loop A for the development of inhibitory small molecules.RNA. 2024 May 16;30(6):609-623. doi: 10.1261/rna.079796.123. RNA. 2024. PMID: 38383158 Free PMC article.
-
Viral RNA structure analysis using DMS-MaPseq.Methods. 2020 Nov 1;183:68-75. doi: 10.1016/j.ymeth.2020.04.001. Epub 2020 Apr 3. Methods. 2020. PMID: 32251733 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

