Alphavirus Replicon DNA Vectors Expressing Ebola GP and VP40 Antigens Induce Humoral and Cellular Immune Responses in Mice
- PMID: 29375526
- PMCID: PMC5767729
- DOI: 10.3389/fmicb.2017.02662
Alphavirus Replicon DNA Vectors Expressing Ebola GP and VP40 Antigens Induce Humoral and Cellular Immune Responses in Mice
Abstract
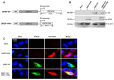
Ebola virus (EBOV) causes severe hemorrhagic fevers in humans, and no approved therapeutics or vaccine is currently available. Glycoprotein (GP) is the major protective antigen of EBOV, and can generate virus-like particles (VLPs) by co-expression with matrix protein (VP40). In this study, we constructed a recombinant Alphavirus Semliki Forest virus (SFV) replicon vector DREP to express EBOV GP and matrix viral protein (VP40). EBOV VLPs were successfully generated and achieved budding from 293 cells after co-transfection with DREP-based GP and VP40 vectors (DREP-GP+DREP-VP40). Vaccination of BALB/c mice with DREP-GP, DREP-VP40, or DREP-GP+DREP-VP40 vectors, followed by immediate electroporation resulted in a mixed IgG subclass production, which recognized EBOV GP and/or VP40 proteins. This vaccination regimen also led to the generation of both Th1 and Th2 cellular immune responses in mice. Notably, vaccination with DREP-GP and DREP-VP40, which produces both GP and VP40 antigens, induced a significantly higher level of anti-GP IgG2a antibody and increased IFN-γ secreting CD8+ T-cell responses relative to vaccination with DREP-GP or DREP-VP40 vector alone. Our study indicates that co-expression of GP and VP40 antigens based on the SFV replicon vector generates EBOV VLPs in vitro, and vaccination with recombinant DREP vectors containing GP and VP40 antigens induces Ebola antigen-specific humoral and cellular immune responses in mice. This novel approach provides a simple and efficient vaccine platform for Ebola disease prevention.
Keywords: Ebola virus; GP and VP40 antigens; SFV replicon vector; immune response; vaccine; virus-like particles.
Figures
Similar articles
-
Distinct Immunogenicity and Efficacy of Poxvirus-Based Vaccine Candidates against Ebola Virus Expressing GP and VP40 Proteins.J Virol. 2018 May 14;92(11):e00363-18. doi: 10.1128/JVI.00363-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514907 Free PMC article.
-
Recombinant Modified Vaccinia Virus Ankara Generating Ebola Virus-Like Particles.J Virol. 2017 May 12;91(11):e00343-17. doi: 10.1128/JVI.00343-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331098 Free PMC article.
-
Potent Anti-hepatitis C Virus (HCV) T Cell Immune Responses Induced in Mice Vaccinated with DNA-Launched RNA Replicons and Modified Vaccinia Virus Ankara-HCV.J Virol. 2019 Mar 21;93(7):e00055-19. doi: 10.1128/JVI.00055-19. Print 2019 Apr 1. J Virol. 2019. PMID: 30674625 Free PMC article.
-
Advances in virus-like particle vaccines for filoviruses.J Infect Dis. 2011 Nov;204 Suppl 3(Suppl 3):S1053-9. doi: 10.1093/infdis/jir346. J Infect Dis. 2011. PMID: 21987741 Free PMC article. Review.
-
Membrane binding and bending in Ebola VP40 assembly and egress.Front Microbiol. 2014 Jun 18;5:300. doi: 10.3389/fmicb.2014.00300. eCollection 2014. Front Microbiol. 2014. PMID: 24995005 Free PMC article. Review.
Cited by
-
Vaccines against Ebola virus and Marburg virus: recent advances and promising candidates.Hum Vaccin Immunother. 2019;15(10):2359-2377. doi: 10.1080/21645515.2019.1651140. Epub 2019 Oct 7. Hum Vaccin Immunother. 2019. PMID: 31589088 Free PMC article. Review.
-
Recombinant PAL/PilE/FlaA DNA vaccine provides protective immunity against Legionella pneumophila in BALB/c mice.BMC Biotechnol. 2020 May 18;20(1):28. doi: 10.1186/s12896-020-00620-3. BMC Biotechnol. 2020. PMID: 32423439 Free PMC article.
-
DNA-launched RNA replicon vaccines induce potent anti-Ebolavirus immune responses that can be further improved by a recombinant MVA boost.Sci Rep. 2018 Aug 20;8(1):12459. doi: 10.1038/s41598-018-31003-6. Sci Rep. 2018. PMID: 30127450 Free PMC article.
-
Application of DNA Replicons in Gene Therapy and Vaccine Development.Pharmaceutics. 2023 Mar 15;15(3):947. doi: 10.3390/pharmaceutics15030947. Pharmaceutics. 2023. PMID: 36986808 Free PMC article. Review.
-
Rational design of self-amplifying virus-like vesicles with Ebola virus glycoprotein as vaccines.Mol Ther. 2024 Oct 2;32(10):3695-3711. doi: 10.1016/j.ymthe.2024.08.026. Epub 2024 Aug 31. Mol Ther. 2024. PMID: 39217415
References
-
- Bates J. T., Pickens J. A., Schuster J. E., Johnson M., Tollefson S. J., Williams J. V., et al. (2016). Immunogenicity and efficacy of alphavirus-derived replicon vaccines for respiratory syncytial virus and human metapneumovirus in nonhuman primates. Vaccine 34 950–956. 10.1016/j.vaccine.2015.12.045 - DOI - PMC - PubMed
-
- Blaney J. E., Marzi A., Willet M., Papaneri A. B., Wirblich C., Feldmann F., et al. (2013). Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLOS Pathog. 9:e1003389. 10.1371/journal.ppat.1003389 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

