Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity
- PMID: 29375543
- PMCID: PMC5770648
- DOI: 10.3389/fimmu.2017.01764
Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity
Abstract
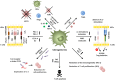
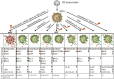
Dendritic cells (DCs) are central players in the initiation and control of responses, regulating the balance between tolerance and immunity. Tolerogenic DCs are essential in the maintenance of central and peripheral tolerance by induction of clonal T cell deletion and T cell anergy, inhibition of memory and effector T cell responses, and generation and activation of regulatory T cells. Therefore, tolerogenic DCs are promising candidates for specific cellular therapy of allergic and autoimmune diseases and for treatment of transplant rejection. Studies performed in rodents have demonstrated the efficacy and feasibility of tolerogenic DCs for tolerance induction in various inflammatory diseases. In the last years, numerous protocols for the generation of human monocyte-derived tolerogenic DCs have been established and some first phase I trials have been conducted in patients suffering from autoimmune disorders, demonstrating the safety and efficiency of this cell-based immunotherapy. This review gives an overview about methods and protocols for the generation of human tolerogenic DCs and their mechanisms of tolerance induction with the focus on interleukin-10-modulated DCs. In addition, we will discuss the prerequisites for optimal clinical grade tolerogenic DC subsets and results of clinical trials with tolerogenic DCs in autoimmune diseases.
Keywords: immunotherapy; nanoparticles; regulatory T cells; tolerance; tolerogenic dendritic cells.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

