The Role of Skin and Orogenital Microbiota in Protective Immunity and Chronic Immune-Mediated Inflammatory Disease
- PMID: 29375574
- PMCID: PMC5767596
- DOI: 10.3389/fimmu.2017.01955
The Role of Skin and Orogenital Microbiota in Protective Immunity and Chronic Immune-Mediated Inflammatory Disease
Abstract
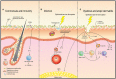
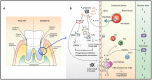
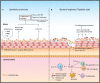
The skin and orogenital mucosae, which constitute complex protective barriers against infection and injuries, are not only the first to come into contact with pathogens but are also colonized by a set of microorganisms that are essential to maintain a healthy physiological environment. Using 16S ribosomal RNA metagenomic sequencing, scientists recognized that the microorganism colonization has greater diversity and variability than previously assumed. These microorganisms, such as commensal bacteria, affect the host's immune response against pathogens and modulate chronic inflammatory responses. Previously, a single pathogen was thought to cause a single disease, but current evidence suggests that dysbiosis of the tissue microbiota may underlie the disease status. Dysbiosis results in aberrant immune responses at the surface and furthermore, affects the systemic immune response. Hence, understanding the initial interaction between the barrier surface immune system and local microorganisms is important for understanding the overall systemic effects of the immune response. In this review, we describe current evidence for the basis of the interactions between pathogens, microbiota, and immune cells on surface barriers and offer explanations for how these interactions may lead to chronic inflammatory disorders.
Keywords: immune response; inflammation; microbiota; orogenital mucosa; skin mucosa.
Figures
Similar articles
-
Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases.Immunol Lett. 2004 May 15;93(2-3):97-108. doi: 10.1016/j.imlet.2004.02.005. Immunol Lett. 2004. PMID: 15158604 Review.
-
Dismicrobism in inflammatory bowel disease and colorectal cancer: changes in response of colocytes.World J Gastroenterol. 2014 Dec 28;20(48):18121-30. doi: 10.3748/wjg.v20.i48.18121. World J Gastroenterol. 2014. PMID: 25561781 Free PMC article. Review.
-
Interactions between intestinal microbiota and innate immune system in pediatric inflammatory bowel disease.J Clin Gastroenterol. 2012 Oct;46 Suppl:S64-6. doi: 10.1097/MCG.0b013e31826a857f. J Clin Gastroenterol. 2012. Retraction in: J Clin Gastroenterol. 2014 Jul;48(6):565. doi: 10.1097/MCG.0000000000000182. PMID: 22955361 Retracted.
-
The role of innate immune signaling in the pathogenesis of atopic dermatitis and consequences for treatments.Semin Immunopathol. 2016 Jan;38(1):29-43. doi: 10.1007/s00281-015-0544-y. Epub 2015 Nov 16. Semin Immunopathol. 2016. PMID: 26573298 Review.
-
The influence of skin microorganisms on cutaneous immunity.Nat Rev Immunol. 2016 May 27;16(6):353-66. doi: 10.1038/nri.2016.48. Nat Rev Immunol. 2016. PMID: 27231051 Review.
Cited by
-
Chlorhexidine Gluconate Bathing Reduces the Incidence of Bloodstream Infections in Adults Undergoing Inpatient Hematopoietic Cell Transplantation.Transplant Cell Ther. 2021 Mar;27(3):262.e1-262.e11. doi: 10.1016/j.jtct.2021.01.004. Epub 2021 Jan 7. Transplant Cell Ther. 2021. PMID: 33781532 Free PMC article.
-
Comparative Analysis of the Microbiome across the Gut-Skin Axis in Atopic Dermatitis.Int J Mol Sci. 2021 Apr 19;22(8):4228. doi: 10.3390/ijms22084228. Int J Mol Sci. 2021. PMID: 33921772 Free PMC article. Review.
-
Microbially competent 3D skin: a test system that reveals insight into host-microbe interactions and their potential toxicological impact.Arch Toxicol. 2020 Oct;94(10):3487-3502. doi: 10.1007/s00204-020-02841-z. Epub 2020 Jul 17. Arch Toxicol. 2020. PMID: 32681188 Free PMC article.
-
[Research progress on the mechanisms of probiotics promoting wound healing].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2024 Jun 25;41(3):635-640. doi: 10.7507/1001-5515.202208003. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2024. PMID: 38932552 Free PMC article. Review. Chinese.
-
A Comprehensive Analysis of Liposomal-Based Nanocarriers for Treating Skin and Soft Tissue Infection.Curr Drug Deliv. 2025;22(5):552-573. doi: 10.2174/0115672018328954240801110200. Curr Drug Deliv. 2025. PMID: 39136517 Review.
References
-
- Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect (2006) 8(8):2195–205. 10.1016/j.micinf.2006.04.001 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

