Differences in Expansion Potential of Naive Chimeric Antigen Receptor T Cells from Healthy Donors and Untreated Chronic Lymphocytic Leukemia Patients
- PMID: 29375575
- PMCID: PMC5767585
- DOI: 10.3389/fimmu.2017.01956
Differences in Expansion Potential of Naive Chimeric Antigen Receptor T Cells from Healthy Donors and Untreated Chronic Lymphocytic Leukemia Patients
Abstract
Introduction: Therapy with chimeric antigen receptor T (CART) cells for hematological malignancies has shown promising results. Effectiveness of CART cells may depend on the ratio of naive (TN) vs. effector (TE) T cells, TN cells being responsible for an enduring antitumor activity through maturation. Therefore, we investigated factors influencing the TN/TE ratio of CART cells.
Materials and methods: CART cells were generated upon transduction of peripheral blood mononuclear cells with a CD19.CAR-CD28-CD137zeta third generation retroviral vector under two different stimulating culture conditions: anti-CD3/anti-CD28 antibodies adding either interleukin (IL)-7/IL-15 or IL-2. CART cells were maintained in culture for 20 days. We evaluated 24 healthy donors (HDs) and 11 patients with chronic lymphocytic leukemia (CLL) for the composition of cell subsets and produced CART cells. Phenotype and functionality were tested using flow cytometry and chromium release assays.
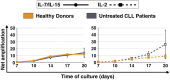
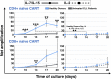
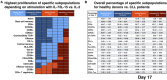
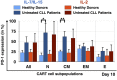
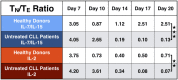
Results: IL-7/IL-15 preferentially induced differentiation into TN, stem cell memory (TSCM: naive CD27+ CD95+), CD4+ and CXCR3+ CART cells, while IL-2 increased effector memory (TEM), CD56+ and CD4+ T regulatory (TReg) CART cells. The net amplification of different CART subpopulations derived from HDs and untreated CLL patients was compared. Particularly the expansion of CD4+ CARTN cells differed significantly between the two groups. For HDs, this subtype expanded >60-fold, whereas CD4+ CARTN cells of untreated CLL patients expanded less than 10-fold. Expression of exhaustion marker programmed cell death 1 on CARTN cells on day 10 of culture was significantly higher in patient samples compared to HD samples. As the percentage of malignant B cells was expectedly higher within patient samples, an excessive amount of B cells during culture could account for the reduced expansion potential of CARTN cells in untreated CLL patients. Final TN/TE ratio stayed <0.3 despite stimulation condition for patients, whereas this ratio was >2 in samples from HDs stimulated with IL-7/IL-15, thus demonstrating efficient CARTN expansion.
Conclusion: Untreated CLL patients might constitute a challenge for long-lasting CART effects in vivo since only a low number of TN among the CART product could be generated. Depletion of malignant B cells before starting CART production might be considered to increase the TN/TE ratio within the CART product.
Keywords: CD19; T cell expansion; T cell subpopulations; chimeric antigen receptor; chronic lymphocytic leukemia; cytokines; immunotherapy; naive T cells.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

