Field evaluation of piglet vaccination with a Mycoplasma hyopneumoniae bacterin as compared to a ready-to-use product including porcine circovirus 2 and M. hyopneumoniae in a conventional French farrow-to-finish farm
- PMID: 29375890
- PMCID: PMC5772722
- DOI: 10.1186/s40813-017-0077-y
Field evaluation of piglet vaccination with a Mycoplasma hyopneumoniae bacterin as compared to a ready-to-use product including porcine circovirus 2 and M. hyopneumoniae in a conventional French farrow-to-finish farm
Abstract
Background: A controlled randomized trial was performed on a well-managed conventional French 180-sow farm. The trial compared the growth performances of piglets vaccinated at weaning (single shot) either with a commercial monovalent Mycoplasma hyopneumoniae bacterin vaccine or with a commercial bivalent vaccine (Porcilis® PCV M Hyo) against M. hyopneumoniae and porcine circovirus 2 (PCV2). The farm's porcine reproductive and respiratory syndrome status was stable, and most diseases (enzootic pneumonia, atrophic rhinitis, post-weaning multisystemic wasting syndrome) were controlled by routine vaccination.
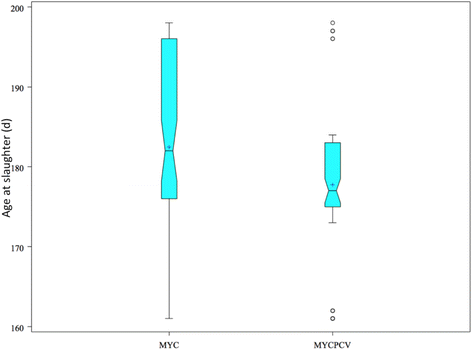
Results: During the post-weaning phase, the growth performances of the piglets vaccinated with the bivalent vaccine were not significantly different from those vaccinated with the monovalent vaccine. However, during the fattening phase the group vaccinated with the bivalent vaccine had a significantly improved ADG (+34 g/d, p = 0. 047), resulting in a 5-day earlier shipment to slaughter. The group also had a shorter and lower PCV2 load in serum during the fattening period, and an improved lung lesions score. In both groups, three pigs died during the peak PCV2 viraemia (16-23 weeks of age). Immunohistochemistry of the lymph nodes showed that in the group vaccinated with the bivalent vaccine, none of these pigs had PCV2-like lesions, while 2 out of the 3 from the other group did. Results suggest that the added PCV2 valence in the vaccination protocol helps countering the negative impact of subclinical PCV2 infection on growth. The calculated return on investment of the added PCV2 vaccine valence was €1.7 extra revenue per slaughtered pig (€ 39 additional revenue per sow and per year), despite the fact that the cost of the bivalent vaccine was higher than the monovalent M. hyopneumoniae vaccine.
Conclusion: In this healthy conventional sow farm, the combined M. hyopneumoniae and PCV2 vaccination was efficacious, convenient to administer and profitable.
Keywords: Mycoplasma hyopneumoniae; PCV2; Randomized controlled field trial; Vaccine.
Conflict of interest statement
A contract was established with the French Pork and Pig Institute (IFIP), owner of the farm, prior to the enrolment of the animals in the study. All blood sampling was performed by the farm’s veterinarian, co-author of this study, in compliance with French animal welfare standards.All authors consent to the publication of the present manuscript.With the exception of the second and third authors, all other authors of this case report are employees of the sponsor company.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Young MG, Cunningham GL, Sanford SE. Circovirus vaccination in pigs with subclinical porcine circovirus type 2 infection complicated by ileitis. J Swine Health Prod. 2011;19(3):175–180.
-
- Fraile L, Grau-Roma L, Sarasola P, Sinovas N, Nofrarías M, López-Jimenez R, López-Soria S, Sibila M, Segalés J. Inactivated PCV2 one shot vaccine applied in 3-week-old piglets: improvement of production parameters and interaction with maternally derived immunity. Vaccine. 2012;30(11):1986–1992. doi: 10.1016/j.vaccine.2012.01.008. - DOI - PubMed
-
- Heißenberger B, Weissenbacher-Lang C, Hennig-Pauka I, Ritzmann M, Ladinig A. Efficacy of vaccination of 3-week-old piglets with Circovac against porcine circovirus diseases (PCVD). Trials Vaccinology 2013;2(2):1-9. http://ac.els-cdn.com/S1879437813000028/1-s2.0-S1879437813000028-main.pd....
LinkOut - more resources
Full Text Sources
Other Literature Sources

