Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals
- PMID: 29375961
- PMCID: PMC5770696
- DOI: 10.1002/advs.201700275
Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals
Abstract
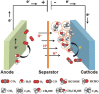
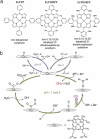
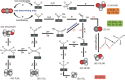
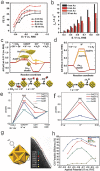
The worldwide unrestrained emission of carbon dioxide (CO2) has caused serious environmental pollution and climate change issues. For the sustainable development of human civilization, it is very desirable to convert CO2 to renewable fuels through clean and economical chemical processes. Recently, electrocatalytic CO2 conversion is regarded as a prospective pathway for the recycling of carbon resource and the generation of sustainable fuels. In this review, recent research advances in electrocatalytic CO2 reduction are summarized from both experimental and theoretical aspects. The referred electrocatalysts are divided into different classes, including metal-organic complexes, metals, metal alloys, inorganic metal compounds and carbon-based metal-free nanomaterials. Moreover, the selective formation processes of different reductive products, such as formic acid/formate (HCOOH/HCOO-), monoxide carbon (CO), formaldehyde (HCHO), methane (CH4), ethylene (C2H4), methanol (CH3OH), ethanol (CH3CH2OH), etc. are introduced in detail, respectively. Owing to the limited energy efficiency, unmanageable selectivity, low stability, and indeterminate mechanisms of electrocatalytic CO2 reduction, there are still many tough challenges need to be addressed. In view of this, the current research trends to overcome these obstacles in CO2 electroreduction field are summarized. We expect that this review will provide new insights into the further technique development and practical applications of CO2 electroreduction.
Keywords: carbon cycle; catalytic mechanisms; electrocatalysts; electrochemical CO2 reduction; renewable fuels.
Figures






References
-
- Shakun J. D., Clark P. U., He F., Marcott S. A., Mix A. C., Liu Z. Y., Otto‐Bliesner B., Schmittner A., Bard E., Nature 2012, 484, 49. - PubMed
-
- Arora V. K., Scinocca J. F., Boer G. J., Christian J. R., Denman K. L., Flato G. M., Kharin V. V., Lee W. G., Merryfield W. J., Geophys. Res. Lett. 2011, 38, 387.
-
- Centi G., Quadrelli E. A., Perathoner S., Energy Environ. Sci. 2013, 6, 1711.
-
- Kondratenko E. V., Mul G., Baltrusaitis J., Larrazábal G. O., Pérez‐Ramírez J., Energy Environ. Sci. 2013, 6, 3112.
-
- Wang W., Wang S. P., Ma X. B., Gong J. L., Chem. Soc. Rev. 2011, 40, 3703. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
