Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design
- PMID: 29375964
- PMCID: PMC5770679
- DOI: 10.1002/advs.201700322
Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design
Abstract
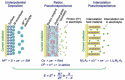
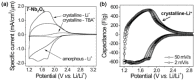
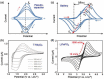
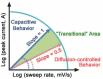
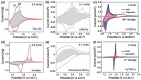
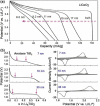
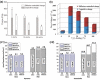
Tremendous efforts have been dedicated into the development of high-performance energy storage devices with nanoscale design and hybrid approaches. The boundary between the electrochemical capacitors and batteries becomes less distinctive. The same material may display capacitive or battery-like behavior depending on the electrode design and the charge storage guest ions. Therefore, the underlying mechanisms and the electrochemical processes occurring upon charge storage may be confusing for researchers who are new to the field as well as some of the chemists and material scientists already in the field. This review provides fundamentals of the similarities and differences between electrochemical capacitors and batteries from kinetic and material point of view. Basic techniques and analysis methods to distinguish the capacitive and battery-like behavior are discussed. Furthermore, guidelines for material selection, the state-of-the-art materials, and the electrode design rules to advanced electrode are proposed.
Keywords: advanced energy storage devices; analytical methods; pseudocapacitance; rational materials design.
Figures






References
-
- Dunn B., Kamath H., Tarascon J.‐M., Science 2011, 334, 928. - PubMed
-
- Yang Z., Zhang J., Kintner‐Meyer M. C. W., Lu X., Choi D., Lemmon J. P., Liu J., Chem. Rev. 2011, 111, 3577. - PubMed
-
- Kim H., Hong J., Park K.‐Y., Kim H., Kim S.‐W., Kang K., Chem. Rev. 2014, 114, 11788. - PubMed
-
- Winter M., Brodd R. J., Chem. Rev. 2004, 104, 4245. - PubMed
-
- Desilvestro J., Haas O., J. Electrochem. Soc. 1990, 137, C5.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
