Pediatric Anaplastic Embryonal Rhabdomyosarcoma: Targeted Therapy Guided by Genetic Analysis and a Patient-Derived Xenograft Study
- PMID: 29376028
- PMCID: PMC5768639
- DOI: 10.3389/fonc.2017.00327
Pediatric Anaplastic Embryonal Rhabdomyosarcoma: Targeted Therapy Guided by Genetic Analysis and a Patient-Derived Xenograft Study
Abstract
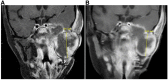
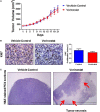
Therapy for rhabdomyosarcoma (RMS) has generally been limited to combinations of conventional cytotoxic agents similar to regimens originally developed in the late 1960s. Recently, identification of molecular alterations through next-generation sequencing of individual tumor specimens has facilitated the use of more targeted therapeutic approaches for various malignancies. Such targeted therapies have revolutionized treatment for some cancer types. However, malignancies common in children, thus far, have been less amenable to such targeted therapies. This report describes the clinical course of an 8-year-old female with embryonal RMS having anaplastic features. This patient experienced multiple relapses after receiving various established and experimental therapies. Genomic testing of this RMS subtype revealed mutations in BCOR, ARID1A, and SETD2 genes, each of which contributes to epigenetic regulation and interacts with or modifies the activity of histone deacetylases (HDAC). Based on these findings, the patient was treated with the HDAC inhibitor vorinostat as a single agent. The tumor responded transiently followed by subsequent disease progression. We also examined the efficacy of vorinostat in a patient-derived xenograft (PDX) model developed using tumor tissue obtained from the patient's most recent tumor resection. The antitumor activity of vorinostat observed with the PDX model reflected clinical observations in that obvious areas of tumor necrosis were evident following exposure to vorinostat. Histologic sections of tumors harvested from PDX tumor-bearing mice treated with vorinostat demonstrated induction of necrosis by this agent. We propose that the evaluation of clinical efficacy in this type of preclinical model merits further evaluation to determine if PDX models predict tumor sensitivity to specific agents and/or combination therapies.
Keywords: anaplastic embryonal rhabdomyosarcoma; case study; patient-derived xenograft; targeted therapy; vorinostat.
Figures
References
-
- Hawkins DS, Anderson JR, Mascarenhas L, McCowage GB, Rodeberg DA, Wolden SL, et al. Vincristine, dactinomycin, cyclophosphamide (VAC) versus VAC/V plus irinotecan (VI) for intermediate-risk rhabdomyosarcoma (IRRMS): a report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. J Clin Oncol (2014) 32:15s; Abstract retrieved from 2014 ASCO Annual Meeting. (No. 10004).10.1200/jco.2014.32.15_suppl.10004 - DOI
-
- Mascarenhas L, Meyer W, Lyden E, Rodeberg D, Indelicato D, Linardic C, et al. Randomized phase II trial of bevacizumab and temsirolimus in combination with vinorelbine (V) and cyclophosphamide (C) for first relapse/disease progression of rhabdomyosarcoma (RMS): a report from the Children’s Oncology Group (COG). J Clin Oncol (2014) 32:15s; Abstract retrieved from 2014 ASCO Annual Meeting. (No. 10003).10.1200/jco.2014.32.15_suppl.10003 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

