Early versus late ureteric stent removal after kidney transplantation
- PMID: 29376218
- PMCID: PMC6491073
- DOI: 10.1002/14651858.CD011455.pub2
Early versus late ureteric stent removal after kidney transplantation
Abstract
Background: Kidney transplantation is the treatment of choice for patients with end-stage kidney disease. In a previous review we concluded that the routine use of ureteric stents in kidney transplantation reduces the incidence of major urological complications (MUC). Unfortunately, this reduction appears to lead to a concomitant rise in urinary tract infections (UTI). For kidney recipients UTI is now the commonest post-transplant complication. This represents a considerable risk to the immunosuppressed transplant recipient, particularly in the era of increased immunologically challenging transplants. There are a number of different approaches taken when considering ureteric stenting and these are associated with differing degrees of morbidity and hospital cost.
Objectives: This review aimed to look at the benefits and harms of early versus late removal of the ureteric stent in kidney transplant recipients.
Search methods: We searched the Cochrane Kidney and Transplant Specialised Register up to 27 March 2017 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register Search Portal and ClinicalTrials.gov.
Selection criteria: All RCTs and quasi-RCTs were included in our meta-analysis. We included recipients of kidney transplants regardless of demography (adults or children) or the type of stent used.
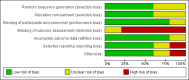
Data collection and analysis: Two authors reviewed the identified studies to ascertain if they met inclusion criteria. We designated removal of a ureteric stent before the third postoperative week (< day 15) or during the index transplant admission as "early" removal. The studies were assessed for quality using the risk of bias tool. The primary outcome of interest was the incidence of MUC. Further outcomes of interest were the incidence of UTI, idiosyncratic stent-related complications, hospital-related costs and adverse events. A subgroup analysis was performed examining the difference in complications reported depending on the type of ureteric stent used; bladder indwelling (BI) versus per-urethral (PU). Statistical analyses were performed using the random effects model and results expressed as relative risk (RR) with 95% confidence intervals (CI).
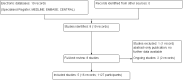
Main results: Five studies (1127 patients) were included in our analysis. Generally the risk of bias of the included studies was judged low or unclear; they addressed the research question and utilised a prospective randomised design. It is uncertain whether early stent removal verus late stent removal improved the incidence of MUC (5 studies, 1127 participants: RR 1.87, 95% CI 0.61 to 5.71; I2 = 21%; low certainty evidence). The incidence of UTI may be reduced in the early stent removal group (5 studies, 1127 participants: RR 0.49 95% CI 0.30 to 0.81; I2 = 59%; moderate certainty evidence). This possible reduction in the UTI incidence was only apparent if a BI stent was used, (3 studies, 539 participants, RR 0.45 95% CI 0.29 to 0.70; I2 = 13%; moderate certainty evidence). However, if an externalised PU stent was used there was no discernible difference in UTI incidence between the early and late group (2 studies, 588 participants: RR 0.60 95% CI 0.17, 2.03; I2 = 83%; low certainty evidence). Data on health economics and quality of life outcomes were lacking.
Authors' conclusions: Early removal of ureteric stents following kidney transplantation may reduce the incidence of UTI while it uncertain if there is a higher risk of MUC. BI stents are the optimum method for achieving this benefit.
Conflict of interest statement
Emily Thompson: none known
Sarah Hosgood: none known
Michael Nicholson: none known
Colin Wilson: none known
Figures
Update of
- doi: 10.1002/14651858.CD011455
Similar articles
-
Routine intraoperative ureteric stenting for kidney transplant recipients.Cochrane Database Syst Rev. 2024 Jul 9;7(7):CD004925. doi: 10.1002/14651858.CD004925.pub4. Cochrane Database Syst Rev. 2024. PMID: 38979749 Free PMC article.
-
Early versus late removal of urinary catheter after kidney transplantation.Cochrane Database Syst Rev. 2023 Jul 14;7(7):CD013788. doi: 10.1002/14651858.CD013788.pub2. Cochrane Database Syst Rev. 2023. PMID: 37449968 Free PMC article.
-
Intermittent catheter techniques, strategies and designs for managing long-term bladder conditions.Cochrane Database Syst Rev. 2021 Oct 26;10(10):CD006008. doi: 10.1002/14651858.CD006008.pub5. Cochrane Database Syst Rev. 2021. PMID: 34699062 Free PMC article.
-
Probiotics for preventing urinary tract infection in people with neuropathic bladder.Cochrane Database Syst Rev. 2017 Sep 8;9(9):CD010723. doi: 10.1002/14651858.CD010723.pub2. Cochrane Database Syst Rev. 2017. PMID: 28884476 Free PMC article.
-
Interventions for increasing immunosuppressant medication adherence in solid organ transplant recipients.Cochrane Database Syst Rev. 2022 Sep 12;9(9):CD012854. doi: 10.1002/14651858.CD012854.pub2. Cochrane Database Syst Rev. 2022. PMID: 36094829 Free PMC article.
Cited by
-
Construction of a nursing solution to prevent and control urinary tract infection in the early stages of kidney transplantation.Transl Androl Urol. 2021 Dec;10(12):4392-4401. doi: 10.21037/tau-21-926. Transl Androl Urol. 2021. PMID: 35070821 Free PMC article.
-
The Impact of Timing of Stent Removal on the Incidence of UTI, Recurrence, Symptomatology, Resistance, and Hospitalization in Renal Transplant Recipients.J Transplant. 2021 Jul 2;2021:3428260. doi: 10.1155/2021/3428260. eCollection 2021. J Transplant. 2021. PMID: 34306740 Free PMC article.
-
Tubeless percutaneous nephrolithotomy (PCNL) for forgotten and retained stent in renal allograft recipient: an interesting case report and lessons learnt.BMJ Case Rep. 2021 Jan 29;14(1):e238438. doi: 10.1136/bcr-2020-238438. BMJ Case Rep. 2021. PMID: 33514620 Free PMC article.
-
A five-year retrospective study focused on urinary tract infections in kidney transplant recipients in the current era of immunosuppression.Front Med (Lausanne). 2025 Jul 22;12:1606224. doi: 10.3389/fmed.2025.1606224. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40766063 Free PMC article.
-
Inhibitory effect of 405 nm laser light on bacterial biofilm in urethral stent.Sci Rep. 2023 Mar 8;13(1):3908. doi: 10.1038/s41598-023-30280-0. Sci Rep. 2023. PMID: 36890147 Free PMC article.
References
References to studies included in this review
Gunawansa 2011 {published data only}
-
- Gunawansa N, Cassim R, Abeydeera A, Wijeyaratne M. Early bedside removal versus late cystoscopic removal of ureteric stents following renal transplantation; Does it make a difference? [abstract no: P‐41]. American Journal of Transplantation 2011;11(Suppl 1):73. [EMBASE: 70329005]
-
- Gunawansa N, Cassim R, Abeydheera A, Wijeyaratne M. Early bedside removal versus late cystoscopic removal of ureteric stents following renal transplantation; does it make a difference? [abstract no: P‐241]. Transplant International 2011;24(Suppl 2):288. [EMBASE: 70528137]
-
- Gunawansa N, Wijeyaratne M, Cassim R, Sahabandu C. Early bedside removal versus delayed cystoscopic removal of ureteric stents following live donor renal transplantation: a randomized prospective study [abstract no: O323]. Transplant International 2015;28(Suppl 4):118. [EMBASE: 72111563]
Huang 2012 {published data only}
-
- Huang L, Wang X, Ma Y, Wang J, Tao X, Liao L, et al. A comparative study of 3‐week and 6‐week duration of double‐J stent placement in renal transplant recipients. Urologia Internationalis 2012;89(1):89‐92. [MEDLINE: ] - PubMed
Indu 2012 {published data only}
Parapiboon 2012 {published data only}
-
- Parapiboon W, Ingsathit A, Disthabanchong S, Nongnuch A, Jearanaipreprem A, Charoenthanakit C, et al. Impact of early ureteric stent removal and cost‐benefit analysis in kidney transplant recipients: results of a randomized controlled study. Transplantation Proceedings 2012;44(3):737‐9. [MEDLINE: ] - PubMed
-
- Parapiboon W, Ingsathit A, Jirasiritham S, Sumethkul V. High incidence of bacteriuria in early post‐kidney transplantation; results from a randomized controlled study. Transplantation Proceedings 2012;44(3):734‐6. [MEDLINE: ] - PubMed
-
- Parapiboon W, Ingsathit A, Junchotikul P, Wiengpon K, Viseshsindh W, Leenanupunth C, et al. Early ureteric stent removal reduces urinary tract infection in kidney transplant recipients, a randomized controlled trial (EUREKA) [abstract no: O‐155]. Transplant International 2011;24(Suppl 2):43. [EMBASE: 70527232]
TrUST 2017 {published data only}
-
- Olsburgh J. Clinical study protocol: TrUST Transplant Ureteric Stent Trial Protocol version 2. 2010. www.isrctn.com/editorial/retrieveFile/81979356‐1fe4‐4b96‐bc2b‐35b7afa4ff... (date accessed 23 August 2017).
-
- Patel P, Rebollo‐Mesa I, Banga N, MacDougall I, Webb M, Mamode N, et al. TrUST (Transplant Ureteric Stent Trial): early versus standard removal [abstract]. European Urology Supplements 2014;13(1):e1010. [EMBASE: 71486000]
-
- Patel P, Rebollo‐Mesa I, Banga N, MacDougall I, Webb M, Mamode N, et al. TrUST (Transplant Ureteric Stent Trial): early versus standard removal [abstract]. Journal of Urology 2014;191(4 Suppl 1):e775‐6. [EMBASE: 71402065]
-
- Patel P, Rebollo‐Mesa I, Banga N, Macdougall I, Webb M, Mamode N, et al. TrUST (Transplant Ureteric Stent Trial): early versus standard removal. A randomised controlled trial [abstract]. Transplantation 2014;98(Suppl 1):638. [EMBASE: 71545698]
-
- Patel P, Rebollo‐Mesa I, Ryan E, Sinha MD, Marks SD, Banga N, et al. Prophylactic ureteric stents in renal transplant recipients: a multicenter randomized controlled trial of early versus late removal. American Journal of Transplantation 2017;17(8):2129‐38. [MEDLINE: ] - PubMed
References to studies excluded from this review
Yari 2014 {published data only}
-
- Yari H, Aliasgari FD, Tara SA, Argani H, Alirezaii A. Benefits and complications of removing the ureteral stent in renal transplantation based on the time interval from transplantation surgery [abstract]. International Journal of Urology 2014;21(Suppl 2):A265. [EMBASE: 71768475]
References to ongoing studies
ACTRN12610000349044 {published data only}
-
- Bartlett A. Pilot study: prospective randomized controlled trial of ureteric JJ stenting with early vs standard stent removal to improve graft and patient outcome and reduce urological complications after renal transplantation. www.anzctr.org.au/ACTRN12610000349044.aspx (first received 30 April 2010).
ISRCTN51276329 {published data only}
-
- Saeb‐Parsy K. A single centre, open label, randomised controlled study to compare the incidence of urinary tract infections and urological complications among renal transplant recipients who have a ureteric stent removed 6‐8 days versus 4‐6 weeks post renal transplantation. www.isrctn.com/ISRCTN51276329 (first received 26 October 2014).
Additional references
Bardapure 2014
-
- Bardapure M, Sharma A, Hammad A. Forgotten ureteric stents in renal transplant recipients: three case reports. Saudi Journal of Kidney Diseases & Transplantation 2014; Vol. 25, issue 1:109‐12. [MEDLINE: ] - PubMed
GRADE 2008
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Montgomery 2012
Morris‐Stiff 1998
Nicholson 1991
Olsburgh 2010
-
- Olsburgh J. Clinical study protocol: TrUST Transplant Ureteric Stent Trial Protocol version 2. 2010. www.isrctn.com/editorial/retrieveFile/81979356‐1fe4‐4b96‐bc2b‐35b7afa4ff... (accessed 23 August 2017).
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Siparsky 2011
-
- Siparsky NF, Kushnir LF, Gallichio MH, Conti DJ. Ureteral stents: a risk factor for polyomavirus BK viremia in kidney transplant recipients undergoing protocol screening. Transplantation Proceedings 2011;43(7):2641‐4. [MEDLINE: ] - PubMed
Thiyagarajan 2012
-
- Thiyagarajan UM, Thiyagarajan P, Bagul A, Nicholson ML. Early removal of ureteric stents and its impact on reducing the urinary infection in renal transplantation‐A single centre experience. www.journal‐surgery.net/article/S1743‐9191(12)00583‐3/pdf (accessed 23 August 2017).
Waters 2008
-
- Waters SL, Heaton K, Siggers JH, Bayston R, Bishop M, Cummings LJ, et al. Ureteric stents: investigating flow and encrustation. Proceedings of the Institution of Mechanical Engineers. Part H ‐ Journal of Engineering in Medicine 2008; Vol. 222, issue 4:551‐61. [MEDLINE: ] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous