Efinaconazole Topical Solution, 10%: Factors Contributing to Onychomycosis Success
- PMID: 29376902
- PMCID: PMC5753103
- DOI: 10.3390/jof1020107
Efinaconazole Topical Solution, 10%: Factors Contributing to Onychomycosis Success
Abstract
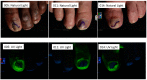
To provide an adequate therapeutic effect against onychomycosis, it has been suggested that topical drugs should have two properties: drug permeability through the nail plate and into the nail bed, and retention of their antifungal activity in the disease-affected areas. Only recently has the importance of other delivery routes (such as subungual) been discussed. Efinaconazole has been shown to have a more potent antifungal activity in vitro than the most commonly used onychomycosis treatments. The low keratin affinity of efinaconazole contributes to its effective delivery through the nail plate and retention of its antifungal activity. Its unique low surface tension formulation provides good wetting properties affording drug delivery both through and under the nail. High antifungal drug concentrations have been demonstrated in the nail of onychomycosis patients, and effectiveness of efinaconazole topical solution, 10% confirmed in two large well-controlled multicenter Phase 3 clinical studies in patients with mild-to-moderate disease.
Keywords: efinaconazole; fungi; onychomycosis; toenail; topical therapy.
Conflict of interest statement
Pollak was an advisor to Valeant Pharmaceuticals and principal investigator in the efinaconazole phase 3 clinical programs. Jo Siu and Pillai are employees of Valeant Pharmaceuticals North America LLC. Tatsumi is an employee of Kaken Pharmaceutical Co. Ltd. (Kyoto, Japan).
Figures

References
-
- Scher R.K., Coppa L.M. Advances in the diagnosis and treatment of onychomycosis. Hosp. Med. 1998;34:11–20.
-
- Murdan S. The nail: Anatomy, physiology, diseases and treatment. In: Murthy S.N., Maibach H.I., editors. Topical Nail Products and Ungual Drug Delivery. CRC Press; Boca Raton, FL, USA: 2013. pp. 1–36.
-
- Sparavigna A., Setaro M., Frisenda L. Physical and microbiological properties of a new nail protective medical device. J. Plastic Dermatol. 2008;4:5–12.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

