Evaluation of a Multimodal, Direct-to-Patient Educational Intervention Targeting Barriers to Osteoporosis Care: A Randomized Clinical Trial
- PMID: 29377378
- PMCID: PMC6016546
- DOI: 10.1002/jbmr.3395
Evaluation of a Multimodal, Direct-to-Patient Educational Intervention Targeting Barriers to Osteoporosis Care: A Randomized Clinical Trial
Abstract
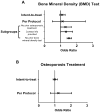
Osteoporosis treatment rates are declining, even among those with past fractures. Novel, low-cost approaches are needed to improve osteoporosis care. We conducted a parallel group, controlled, randomized clinical trial evaluating a behavioral intervention for improving osteoporosis medication use. A total of 2684 women with self-reported fracture history after age 45 years not using osteoporosis therapy from US Global Longitudinal Study of Osteoporosis in Women (GLOW) sites were randomized 1:1 to receive a multimodal, tailored, direct-to-patient, video intervention versus usual care. The primary study outcome was self-report of osteoporosis medication use at 6 months. Other outcomes included calcium and vitamin D supplementation, bone mineral density (BMD) testing, readiness for behavioral change, and barriers to treatment. In intent-to-treat analyses, there were no significant differences between groups (intervention versus control) in osteoporosis medication use (11.7% versus 11.4%, p = 0.8), calcium supplementation (31.8% versus 32.6%, p = 0.7), vitamin D intake (41.3% versus 41.9%, p = 0.8), or BMD testing (61.8% versus 57.1%, p = 0.2). In the intervention group, fewer women were in the precontemplative stage of behavior change, more women reported seeing their primary care provider, had concerns regarding osteonecrosis of the jaw, and difficulty in taking/remembering to take osteoporosis medications. We found differences in BMD testing among the subgroup of women with no prior osteoporosis treatment, those who provided contact information, and those with no past BMD testing. In per protocol analyses, women with appreciable exposure to the online intervention (n = 257) were more likely to start nonbisphosphonates (odds ratio [OR] = 2.70; 95% confidence interval [CI] 1.26-5.79) compared with the usual care group. Although our intervention did not increase the use of osteoporosis therapy at 6 months, it increased nonbisphosphonate medication use and BMD testing in select subgroups, shifted participants' readiness for behavior change, and altered perceptions of barriers to osteoporosis treatment. Achieving changes in osteoporosis care using patient activation approaches alone is challenging. © 2018 American Society for Bone and Mineral Research.
Trial registration: ClinicalTrials.gov NCT01907269.
Keywords: BEHAVIORAL INTERVENTION; BISPHOSPHONATES; FRACTURE PREVENTION; NONBISPHOSPHONATES; OSTEOPOROSIS.
© 2018 American Society for Bone and Mineral Research.
Figures
References
-
- Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85(11):4118–24. - PubMed
-
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. - PubMed
-
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. The New England journal of medicine. 2007;356(18):1809–22. - PubMed
-
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–52. - PubMed
-
- National Committee for Quality Assurance. [Accessed on October 28, 2010];HEDIS & Quality Measurement. Available at http://www.ncqa.org/tabid/59/Default.aspx.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

