Association of Vibrio cholerae 569B outer membrane vesicles with host cells occurs in a GM1-independent manner
- PMID: 29377560
- PMCID: PMC5980675
- DOI: 10.1111/cmi.12828
Association of Vibrio cholerae 569B outer membrane vesicles with host cells occurs in a GM1-independent manner
Abstract
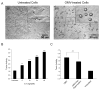
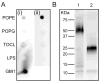
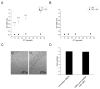
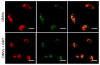
The primary virulence factor of Vibrio cholerae, cholera toxin (CT), initiates a pathway in epithelial cells that leads to the severe diarrhoea characteristic of cholera. Secreted CT binds to GM1 on the surface of host cells to facilitate internalisation. Many bacterial toxins, including CT, have been shown to be additionally delivered via outer membrane vesicles (OMVs). A fraction of the closely related heat labile toxin produced by enterotoxigenic Escherichia coli has been demonstrated to reside on the surface of OMVs, where it binds GM1 to facilitate OMV internalisation by host cells. In this work, we investigated whether OMV-associated CT is likewise trafficked to host cells in a GM1-dependent mechanism. We demonstrated that a majority of CT is secreted in its OMV-associated form and is located exclusively inside the vesicle. Therefore, the toxin is unable to bind GM1 on the host cell surface, and the OMVs are trafficked to the host cells in a GM1-independent mechanism. These findings point to a secondary, noncompeting mechanism for secretion and delivery of CT, beyond its well-studied secretion via a Type II secretion system and underscore the importance of focusing future studies on understanding this GM1-independent delivery mechanism to fully understand Vibrio cholerae pathogenesis.
© 2018 John Wiley & Sons Ltd.
Figures



Similar articles
-
Outer Membrane Vesicles of Vibrio cholerae Protect and Deliver Active Cholera Toxin to Host Cells via Porin-Dependent Uptake.mBio. 2021 Jun 29;12(3):e0053421. doi: 10.1128/mBio.00534-21. Epub 2021 May 26. mBio. 2021. PMID: 34076466 Free PMC article.
-
Cholera Toxin Encapsulated within Several Vibrio cholerae O1 Serotype Inaba Outer Membrane Vesicles Lacks a Functional B-Subunit.Toxins (Basel). 2019 Apr 6;11(4):207. doi: 10.3390/toxins11040207. Toxins (Basel). 2019. PMID: 30959895 Free PMC article.
-
Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells.FEBS Lett. 2011 May 6;585(9):1357-62. doi: 10.1016/j.febslet.2011.04.017. Epub 2011 Apr 14. FEBS Lett. 2011. PMID: 21510946
-
Cholera: pathophysiology and emerging therapeutic targets.Future Med Chem. 2013 May;5(7):781-98. doi: 10.4155/fmc.13.42. Future Med Chem. 2013. PMID: 23651092 Review.
-
Role of membrane gangliosides in the binding and action of bacterial toxins.J Membr Biol. 1982;69(2):85-97. doi: 10.1007/BF01872268. J Membr Biol. 1982. PMID: 6752418 Review.
Cited by
-
Bacterial Outer Membrane Vesicles as Antibiotic Delivery Vehicles.Front Immunol. 2021 Sep 20;12:733064. doi: 10.3389/fimmu.2021.733064. eCollection 2021. Front Immunol. 2021. PMID: 34616401 Free PMC article. Review.
-
Characterization of the Inflammatory Response Evoked by Bacterial Membrane Vesicles in Intestinal Cells Reveals an RIPK2-Dependent Activation by Enterotoxigenic Escherichia coli Vesicles.Microbiol Spectr. 2023 Aug 17;11(4):e0111523. doi: 10.1128/spectrum.01115-23. Epub 2023 Jun 12. Microbiol Spectr. 2023. PMID: 37306596 Free PMC article.
-
Heterogeneity of Size and Toxin Distribution in Aggregatibacter actinomycetemcomitans Outer Membrane Vesicles.Toxins (Basel). 2024 Mar 7;16(3):138. doi: 10.3390/toxins16030138. Toxins (Basel). 2024. PMID: 38535804 Free PMC article.
-
Inhibition of bacterial toxin recognition of membrane components as an anti-virulence strategy.J Biol Eng. 2019 Feb 19;13:4. doi: 10.1186/s13036-018-0138-z. eCollection 2019. J Biol Eng. 2019. PMID: 30820243 Free PMC article. Review.
-
Delivery of Toxins and Effectors by Bacterial Membrane Vesicles.Toxins (Basel). 2021 Nov 26;13(12):845. doi: 10.3390/toxins13120845. Toxins (Basel). 2021. PMID: 34941684 Free PMC article. Review.
References
-
- Balakrishnan VS. Cholera in Yemen. Elsevier; 2017. - PubMed
-
- Balsalobre C, Silván JM, Berglund S, Mizunoe Y, Uhlin BE, Wai SN. Release of the type I secreted α-haemolysin via outer membrane vesicles from Escherichia coli. Molecular Microbiology. 2006;59(1):99–112. - PubMed
-
- Bielaszewska M, Ruter C, Bauwens A, Greune L, Jarosch KA, Steil D, … Karch H. Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic Escherichia coli O157: Intracellular delivery, trafficking and mechanisms of cell injury. Plos Pathogens. 2017;13(2):51. doi: 10.1371/journal.ppat.1006159. - DOI - PMC - PubMed
-
- Bielaszewska M, Ruter C, Kunsmann L, Greune L, Bauwens A, Zhang WL, … Karch H. Enterohemorrhagic Escherichia coli Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis. Plos Pathogens. 2013;9(12):30. doi: 10.1371/journal.ppat.1003797. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

