Hepatitis B virus core protein allosteric modulators can distort and disrupt intact capsids
- PMID: 29377794
- PMCID: PMC5788503
- DOI: 10.7554/eLife.31473
Hepatitis B virus core protein allosteric modulators can distort and disrupt intact capsids
Abstract
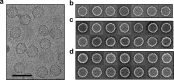
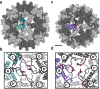
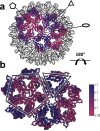
Defining mechanisms of direct-acting antivirals facilitates drug development and our understanding of virus function. Heteroaryldihydropyrimidines (HAPs) inappropriately activate assembly of hepatitis B virus (HBV) core protein (Cp), suppressing formation of virions. We examined a fluorophore-labeled HAP, HAP-TAMRA. HAP-TAMRA induced Cp assembly and also bound pre-assembled capsids. Kinetic and spectroscopic studies imply that HAP-binding sites are usually not available but are bound cooperatively. Using cryo-EM, we observed that HAP-TAMRA asymmetrically deformed capsids, creating a heterogeneous array of sharp angles, flat regions, and outright breaks. To achieve high resolution reconstruction (<4 Å), we introduced a disulfide crosslink that rescued particle symmetry. We deduced that HAP-TAMRA caused quasi-sixfold vertices to become flatter and fivefold more angular. This transition led to asymmetric faceting. That a disordered crosslink could rescue symmetry implies that capsids have tensegrity properties. Capsid distortion and disruption is a new mechanism by which molecules like the HAPs can block HBV infection.
Keywords: biochemistry; cryo-EM; direct acting antiviral; hepatitis B virus; image reconstruction; infectious disease; microbiology; self-assembly; virus.
© 2017, Schlicksup et al.
Conflict of interest statement
CS AZ has an interest in Assembly Biosciences, a company that is pursuing antivirals directed against HBV, JW, SF, WT, AZ No competing interests declared, BV SF is an employee of Assembly Biosciences, a company that is pursuing antivirals directed against HBV, MV WW is an employee of Assembly Biosciences, a company that is pursuing antivirals directed against HBV
Figures




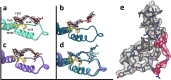



References
-
- Adachi K, Watanabe K, Yamazaki S. pH-responsive switchable aggregation phenomena of xanthene dyes adsorbed on tungsten(VI) oxide colloid surface. Industrial & Engineering Chemistry Research. 2014;53:13046–13057. doi: 10.1021/ie5018817. - DOI
-
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica Section D Biological Crystallography. 2012;68:352–367. doi: 10.1107/S0907444912001308. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

