Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy
- PMID: 29378114
- PMCID: PMC5834400
- DOI: 10.1021/acsnano.7b05876
Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy
Abstract
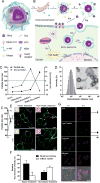
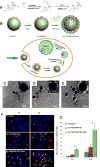
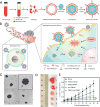
The immune cytokine tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has received significant attention as a cancer therapeutic due to its ability to selectively trigger cancer cell apoptosis without causing toxicity in vivo. While TRAIL has demonstrated significant promise in preclinical studies in mice as a cancer therapeutic, challenges including poor circulation half-life, inefficient delivery to target sites, and TRAIL resistance have hindered clinical translation. Recent advances in drug delivery, materials science, and nanotechnology are now being exploited to develop next-generation nanoparticle platforms to overcome barriers to TRAIL therapeutic delivery. Here, we review the design and implementation of nanoparticles to enhance TRAIL-based cancer therapy. The platforms we discuss are diverse in their approaches to the delivery problem and provide valuable insight into guiding the design of future nanoparticle-based TRAIL cancer therapeutics to potentially enable future translation into the clinic.
Keywords: biological barriers; biomaterials; cancer therapy; drug delivery; gene therapy; immunotherapy; metastasis; nanotechnology; oncology; tumor targeting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Nanocarriers for TRAIL delivery: driving TRAIL back on track for cancer therapy.Nanoscale. 2017 Sep 28;9(37):13879-13904. doi: 10.1039/c7nr04959e. Nanoscale. 2017. PMID: 28914952 Review.
-
Synergistic anti-cancer effects via co-delivery of TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) and doxorubicin using micellar nanoparticles.Mol Biosyst. 2011 May;7(5):1512-22. doi: 10.1039/c0mb00266f. Epub 2011 Feb 24. Mol Biosyst. 2011. PMID: 21350763
-
Presentation and Delivery of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand via Elongated Plant Viral Nanoparticle Enhances Antitumor Efficacy.ACS Nano. 2019 Feb 26;13(2):2501-2510. doi: 10.1021/acsnano.8b09462. Epub 2019 Jan 22. ACS Nano. 2019. PMID: 30668110
-
Combination Nanopreparations of a Novel Proapoptotic Drug - NCL-240, TRAIL and siRNA.Pharm Res. 2016 Jul;33(7):1587-601. doi: 10.1007/s11095-016-1899-z. Epub 2016 Mar 7. Pharm Res. 2016. PMID: 26951567
-
NanoTRAIL-Oncology: A Strategic Approach in Cancer Research and Therapy.Adv Healthc Mater. 2018 Jun;7(11):e1800053. doi: 10.1002/adhm.201800053. Epub 2018 Mar 12. Adv Healthc Mater. 2018. PMID: 29527836 Review.
Cited by
-
TLR2 and TLR9 Blockade Using Specific Intrabodies Inhibits Inflammation-Mediated Pancreatic Cancer Cell Growth.Antibodies (Basel). 2024 Feb 1;13(1):11. doi: 10.3390/antib13010011. Antibodies (Basel). 2024. PMID: 38390872 Free PMC article.
-
Gadolinium-hyaluronic acid nanoparticles as an efficient and safe magnetic resonance imaging contrast agent for articular cartilage injury detection.Bioact Mater. 2020 Jun 21;5(4):758-767. doi: 10.1016/j.bioactmat.2020.05.009. eCollection 2020 Dec. Bioact Mater. 2020. PMID: 32637740 Free PMC article.
-
TRAIL of Hope Meeting Resistance in Cancer.Trends Cancer. 2020 Dec;6(12):989-1001. doi: 10.1016/j.trecan.2020.06.006. Epub 2020 Jul 24. Trends Cancer. 2020. PMID: 32718904 Free PMC article. Review.
-
Delivery technologies to engineer natural killer cells for cancer immunotherapy.Cancer Gene Ther. 2021 Sep;28(9):947-959. doi: 10.1038/s41417-021-00336-2. Epub 2021 Apr 22. Cancer Gene Ther. 2021. PMID: 33888870 Review.
-
Double-Edged Lipid Nanoparticles Combining Liposome-Bound TRAIL and Encapsulated Doxorubicin Showing an Extraordinary Synergistic Pro-Apoptotic Potential.Cancers (Basel). 2019 Dec 5;11(12):1948. doi: 10.3390/cancers11121948. Cancers (Basel). 2019. PMID: 31817469 Free PMC article.
References
-
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New Aspects of Natural-Killer-Cell Surveillance and Therapy of Cancer. Nat. Rev. Cancer. 2002;2:850–861. - PubMed
-
- Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Surveillance of Tumor Metastasis by Liver Natural Killer Cells. Nat. Med. 2001;7:94–100. - PubMed
-
- Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased Susceptibility to Tumor Initiation and Metastasis in TNF-Related Apoptosis-Inducing Ligand-Deficient Mice. J. Immunol. 2002;168:1356–1361. - PubMed
-
- Johnstone RW, Frew AJ, Smyth MJ. The TRAIL Apoptotic Pathway in Cancer Onset, Progression and Therapy. Nat. Rev. Cancer. 2008;8:782–798. - PubMed
-
- Plasilova M, Zivny J, Jelinek J, Neuwirtova R, Cermak J, Necas E, Andera L, Stopka T. TRAIL (Apo2L) Suppresses Growth of Primary Human Leukemia and Myelodysplasia Progenitors. Leukemia. 2002;16:67–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

