Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals
- PMID: 29378131
- PMCID: PMC5908184
- DOI: 10.1021/jacs.7b13477
Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals
Abstract
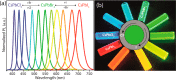
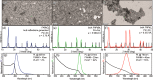
We propose here a new colloidal approach for the synthesis of both all-inorganic and hybrid organic-inorganic lead halide perovskite nanocrystals (NCs). The main limitation of the protocols that are currently in use, such as the hot injection and the ligand-assisted reprecipitation routes, is that they employ PbX2 (X = Cl, Br, or I) salts as both lead and halide precursors. This imposes restrictions on being able to precisely tune the amount of reaction species and, consequently, on being able to regulate the composition of the final NCs. In order to overcome this issue, we show here that benzoyl halides can be efficiently used as halide sources to be injected in a solution of metal cations (mainly in the form of metal carboxylates) for the synthesis of APbX3 NCs (in which A = Cs+, CH3NH3+, or CH(NH2)2+). In this way, it is possible to independently tune the amount of both cations and halide precursors in the synthesis. The APbX3 NCs that were prepared with our protocol show excellent optical properties, such as high photoluminescence quantum yields, low amplified spontaneous emission thresholds, and enhanced stability in air. It is noteworthy that CsPbI3 NCs, which crystallize in the cubic α phase, are stable in air for weeks without any postsynthesis treatment. The improved properties of our CsPbX3 perovskite NCs can be ascribed to the formation of lead halide terminated surfaces, in which Cs cations are replaced by alkylammonium ions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

